Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In systemic lupus erythematosus (SLE), loss of self-tolerance and pathologic T and B cell interactions lead to autoantibodies against nuclear antigens (ANA) production. In past cohorts, 23-57% of those with suspected SLE progress to definite SLE, but it is difficult to identify those who will, as accurate prognostic biomarkers are lacking. We hypothesized that cellular features of immune activation would identify patients progressing to SLE, allowing earlier diagnosis and ultimately damage prevention.
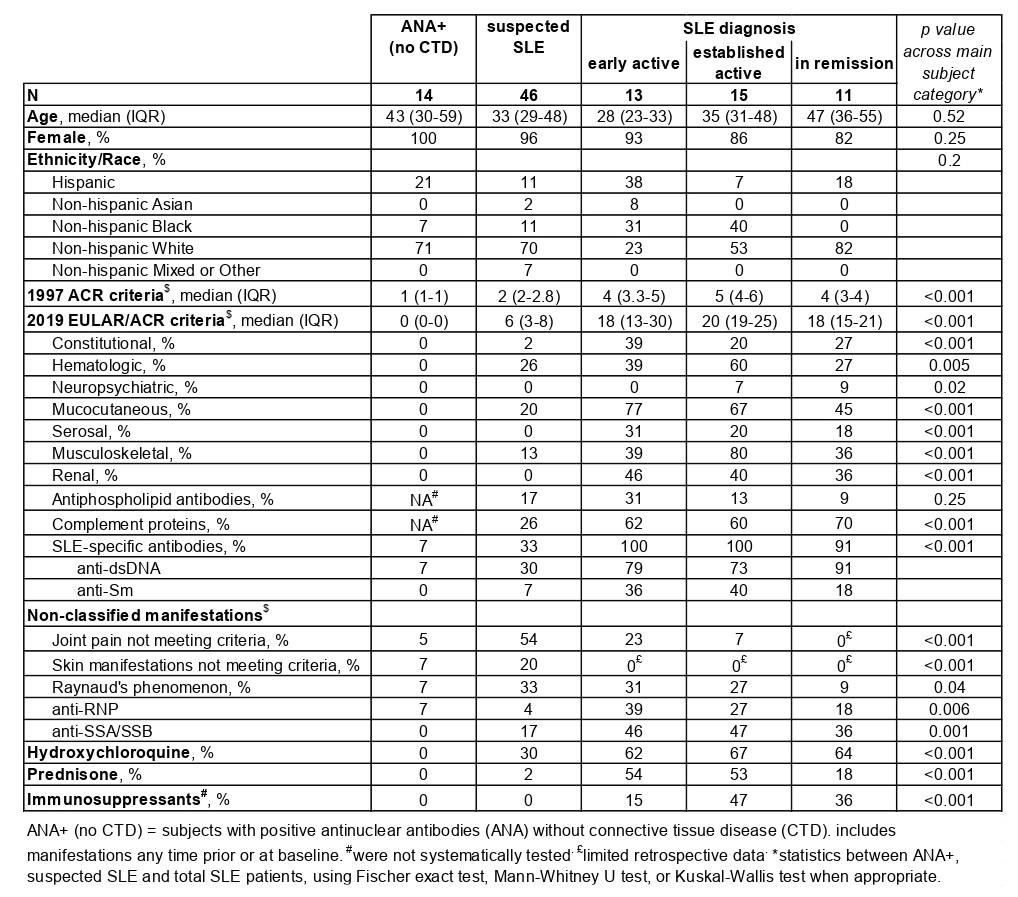
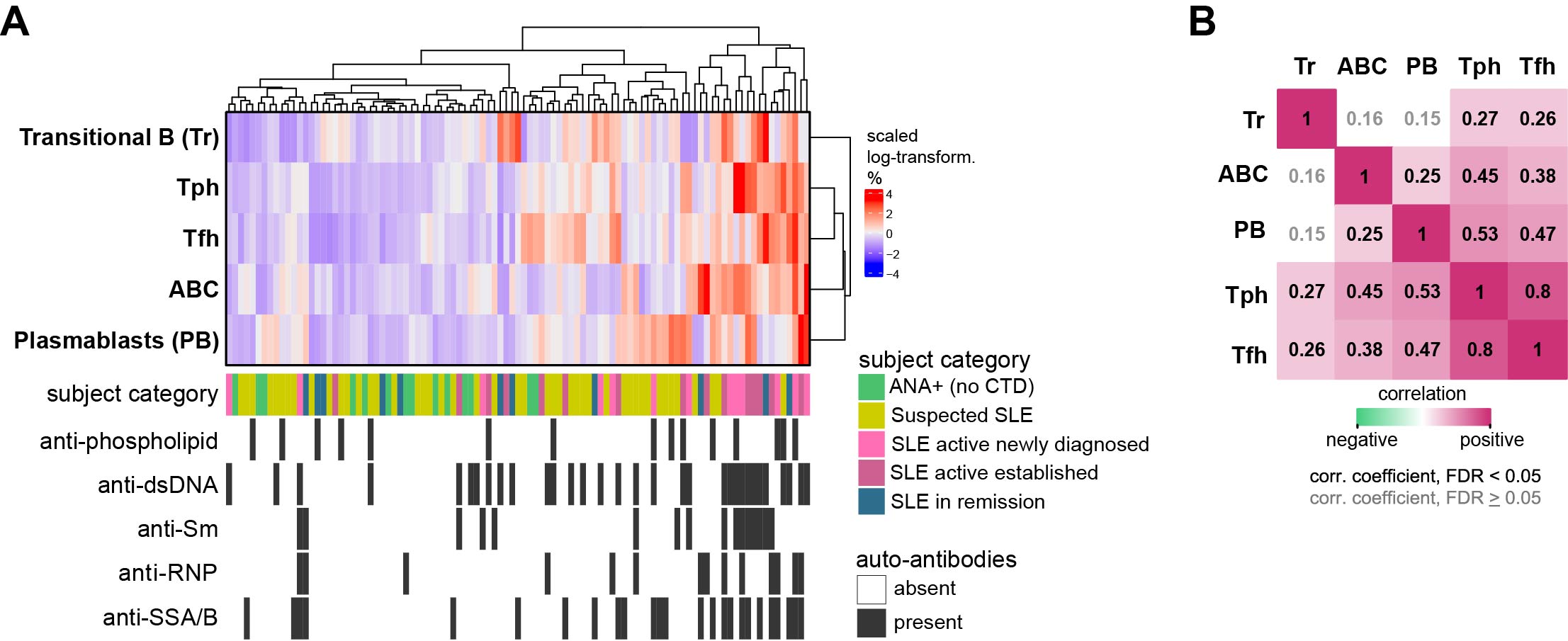
Methods: Blood samples were collected longitudinally (~every 6 mos) from ANA+ patients with suspected SLE (suspicion or new symptoms since < 3 yrs). At baseline, subjects did not meet any SLE classification criteria and received prednisone < 10mg/day and no immunosuppressants. ANA+ patients without any suspicion of connective tissue disease also had blood drawn, as did patients diagnosed and meeting EULAR/ACR 2019 SLE criteria, in 3 sets: a) new SLE < 1 year with active disease, b) established SLE > 1 year with active disease, and 3) SLE in remission for ≥ 2 years; excluding those receiving belimumab or rituximab. Flow cytometry on whole blood (< 12h after collection) quantified 5 lymphocyte subsets previously associated with immune activation in SLE: transitional B cells (Tr, CD38hiCD27-), ABC (CD27-CD38lowCD21lowCD11c+), plasmablasts (CD27+CD38hi), Tph cells (CXCR5-ICOS+PD-1hi), and Tfh cells (CXCR5+ICOS+PD-1hi). We calculated a composite Lupus Lymphocyte Activation Score (LLAS) by combining normalized proportions of these 5 cell subsets.
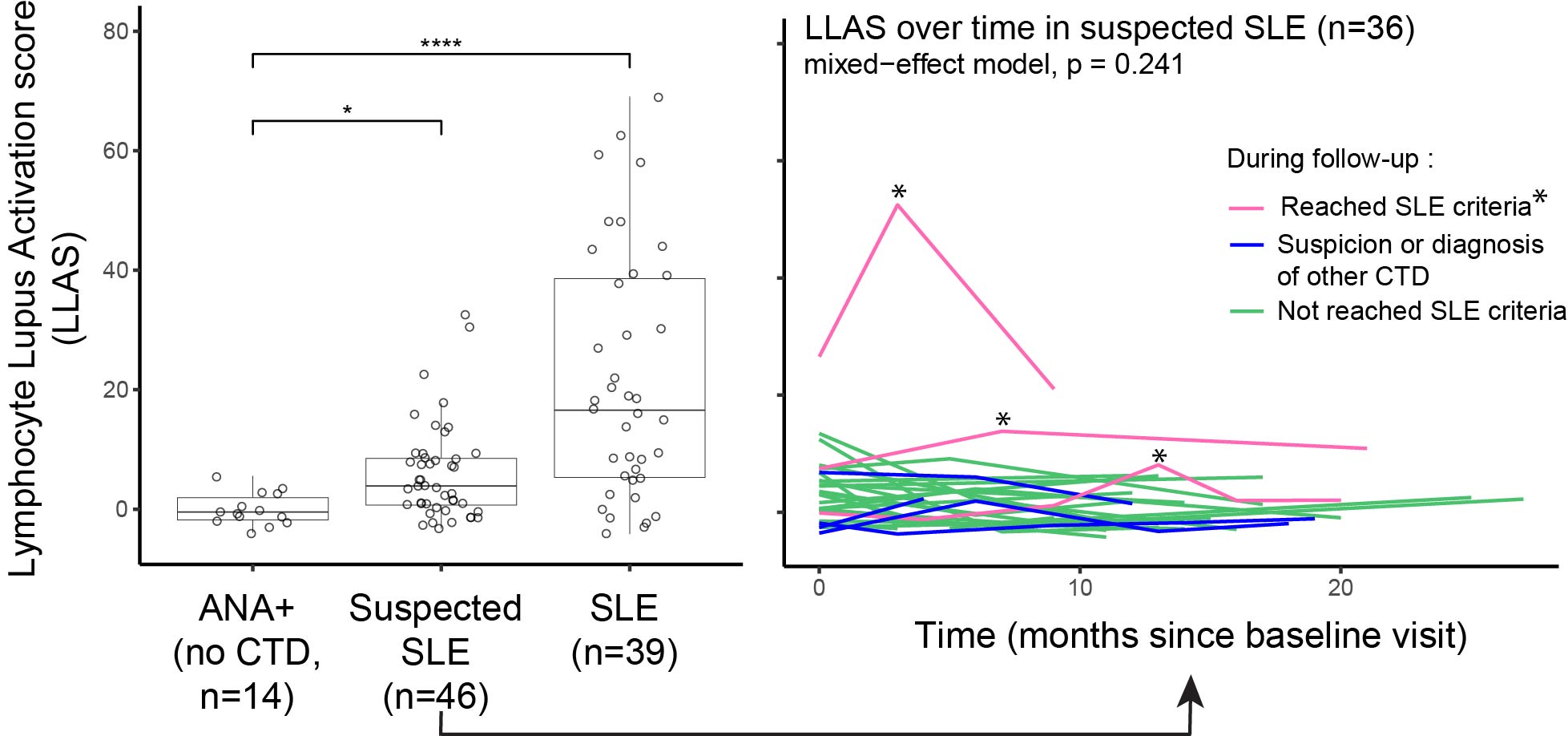
Results: We studied 145 samples: 103 from 46 patients with suspected SLE, 14 from ANA+ individuals, and 39 from patients with definite SLE (13 with active new SLE, 15 with active established disease, and 11 in remission) (Table 1 Baseline characteristics). 36 of the 46 patients with suspected SLE had multiple visits, mean (SD) follow-up 13.2 (5.6) months. In follow-up, 3 patients were diagnosed and met 2012 SLICC or 2019 EULAR/ACR criteria; 4 patients were diagnosed or suspected of having Sjögren’s syndrome, dermatomyositis, seronegative rheumatoid arthritis or spondylarthritis. To examine patterns of T/B cell proportions, we used hierarchical clustering and identified a gradient ranging from lowest proportions of all 5 cell subsets among ANA+ patients to the highest among active SLE patients with multiple autoantibodies (Figure 1A). Those with suspected SLE were widely distributed in the hierarchical clustering (Figure 1A). In the heatmap gradient and autoantibody-associated patterns, Tph and Tfh cells strongly correlated with ABCs and plasmablasts (Figure 1B). Patients with suspected SLE had significantly higher LLAS than ANA+ patients, yet with a broad range (Figure 2A). The 3 patients who developed classified SLE had either the highest or most increased LLAS in follow-up (Figure 2B).
Conclusion: Using flow cytometry with limited markers, we defined a LLAS score capturing key lymphocyte subsets implicated in SLE immune dysregulation. LLAS was highly expanded in SLE patients and in patients developing SLE (vs. ANA+ only patients), suggesting a role for LLAS as an early SLE biomarker.
To cite this abstract in AMA style:
Horisberger A, Adejorin I, Caldropoli J, Dillon E, Oakes E, Marks K, Sasaki T, Costenbader K, Rao D. Lupus Lymphocyte Activation Score of Pathologic T and B Cell Phenotypes Increased in Patients at Risk of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/lupus-lymphocyte-activation-score-of-pathologic-t-and-b-cell-phenotypes-increased-in-patients-at-risk-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-lymphocyte-activation-score-of-pathologic-t-and-b-cell-phenotypes-increased-in-patients-at-risk-of-systemic-lupus-erythematosus/