Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Up to 80% of RA patients use glucocorticoids (GC) at some time in their illness. Current ACR guidelines note that difficulty tapering GC promotes chronic use despite an unfavorable toxicity profile, and recommend escalating DMARDs over GC continuation to maintain low disease activity or remission (LDA). In the recent Steroid EliMination In RA (SEMIRA) trial, 65% of RA patients in LDA on stable biologic therapy successfully tapered GCs. We aimed to evaluate real-world rates of GC tapering among similar patients enrolled in the VA Rheumatoid Arthritis (VARA) registry.
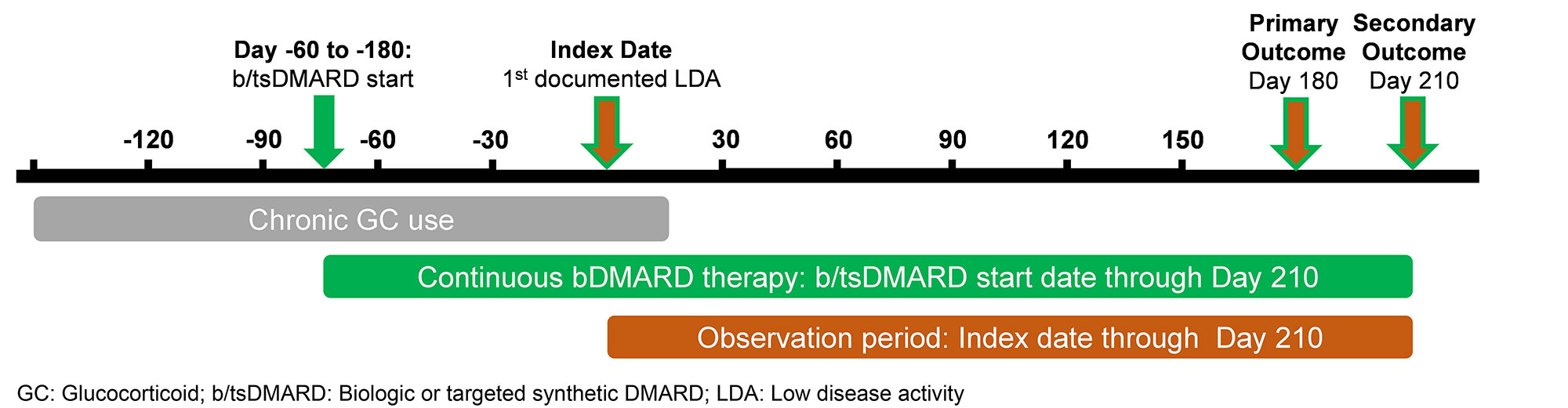
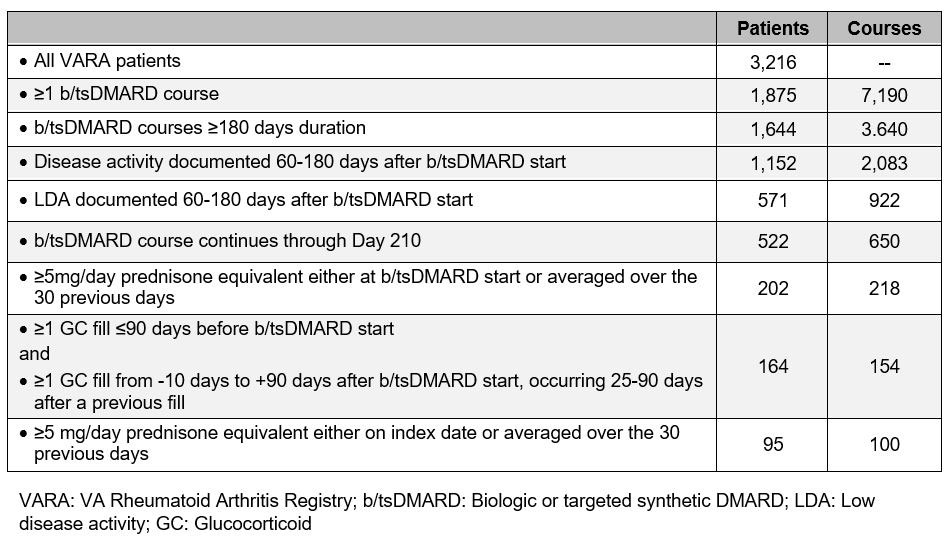
Methods: We linked the VARA registry, a multicenter prospective cohort of US Veterans with RA, to national VA pharmacy claims from 2003-2022 to identify chronic GC users who achieved LDA after starting a new biologic or targeted synthetic DMARD (b/tsDMARD) (Fig. 1). We defined index date as the first recorded LDA occurring 60-180 days after the first fill of the b/tsDMARD fill. We conceived of “chronic GC users” as patients on GC before b/tsDMARD initiation, who continued them until they achieved LDA. Our algorithm defining chronic GC use is outlined in Table 1. Our primary outcome, assessed at Day 180, required a) 50% reduction in 30-day averaged GC dose compared to index date; b) 30-day averaged GC dose ≤5mg/day. Our secondary outcome was GC discontinuation, defined as 30-day averaged GC dose of 0mg/day at both Day 180 and Day 210. We used univariate statistics to compare patient characteristics across a) all meeting inclusion criteria; b) those achieving the primary outcome; c) those not achieving the primary outcome.
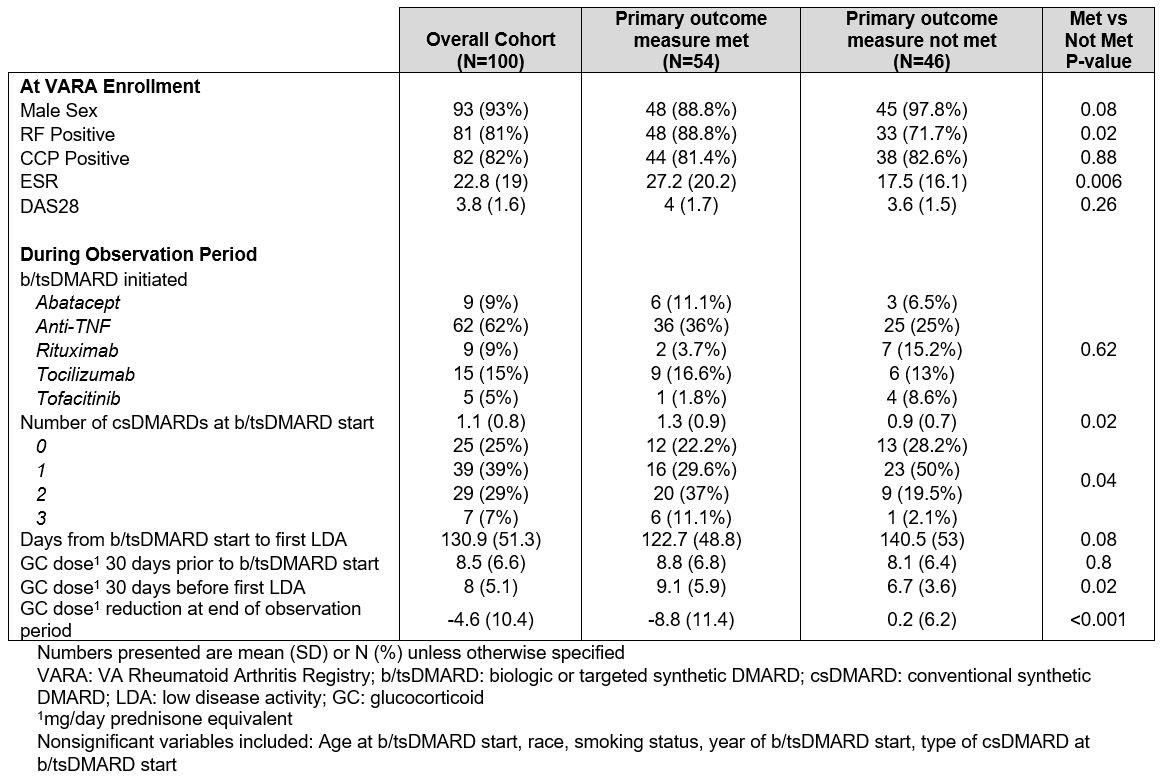
Results: Of 3,216 VARA participants, 100 b/tsDMARD courses among 95 patients (3%) met inclusion criteria and were evaluated (Table 2). Of these, 54 courses met the primary outcome, and 33 resulted in GC discontinuation. Table 2 presents patient characteristics by course. Cohort demographics reflect those of the larger VARA population. The b/tsDMARD was a TNF inhibitor in 62% of cases. Common background DMARDs included MTX (40%), HCQ (39%), SSZ (14%) and LEF (14%); 46% of patients were on ≥2 conventional synthetic DMARDs (csDMARDs) at b/tsDMARD start. Mean prednisone dose at b/tsDMARD start was 8.5mg. Mean time from b/tsDMARD start to LDA was 131 days. Female sex, positive RF, enrollment ESR, use of more csDMARDs at b/tsDMARD start, shorter time from b/tsDMARD start to index date, and higher GC dose 30 days before index date were positively associated with the primary outcome (Table 2).
Conclusion: In a real-world population of chronic GC users achieving LDA after starting a new b/tsDMARD for RA, 54% of courses achieved a 50% reduction in average GC dose, and 33% achieved discontinuation. While only a limited number of registry patients met inclusion criteria, our findings are consistent with prior observational data, and with the SEMIRA trial results. Concurrent use of background csDMARDs, enrollment ESR, and GC dose 30 days prior to LDA were associated with the primary outcome, while type of b/tsDMARD initiated, and GC dose prior to b/tsDMARD start were not. Future work will validate the claims-based definitions used to assess GC use and tapering, and evaluate claims and registry-based predictors of successful and unsuccessful GC tapering.
To cite this abstract in AMA style:
Wallace B, England B, Baker J, Kunkel G, Braaten T, Rojas J, Petro A, Roul P, Mikuls T, Sauer B, Cannon G. Lowering Expectations: Glucocorticoid Tapering Among Veterans with Rheumatoid Arthritis Achieving Low Disease Activity on Stable Biologic Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/lowering-expectations-glucocorticoid-tapering-among-veterans-with-rheumatoid-arthritis-achieving-low-disease-activity-on-stable-biologic-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lowering-expectations-glucocorticoid-tapering-among-veterans-with-rheumatoid-arthritis-achieving-low-disease-activity-on-stable-biologic-therapy/