Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: To limit the risk of serious infections, guidelines recommend short term (< 3 months) or low-dose (≤10 mg/day) adjunct glucocorticoids (GCs) to control rheumatoid arthritis (RA) flares or high disease activity1. In the ICHIBAN study, which analysed the safety and effectiveness of tocilizumab (TCZ), similar proportions of patients with (61.2%) and without (62.5%) concomitant baseline (BL) GCs achieved DAS28-ESR remission2. Here, we examine the efficacy and safety of TCZ in subgroups according to BL concomitant GCs in detail.
Methods: ICHIBAN was a prospective, multi-center, non-interventional study (NCT01194401; ML22928) that included adults with active, moderate-to-severe RA who received TCZ according to the local label in Germany. All patients who received ≥1 TCZ infusion were included in the safety population (SAF, n=3164). Effectiveness (DAS28-ESR and CDAI) and concomitant medication were analysed in all SAF patients with no prior TCZ (n=2902).
Results: In ICHIBAN, 2,550 (80.6%) patients received concomitant GCs, while 607 (19.2%) received no GCs (7 missing) at BL. Patients with or without GCs were of similar mean [SD] age (55.9 [13.0] vs 53.8 [13.6] years old). The proportion of female patients was numerically lower in the group with BL GCs (73.4%) than in those without GCs (80.9%). A numerically greater proportion of patients with BL GCs (73.1%) had comorbidities than those without (67.9%). Common comorbidities in patients with and without GCs were hypertension (37.4% vs 35.1%), degenerative joint disorder/spinal disease (20.4% vs 14.2%), and osteoporosis (19.1% vs 9.1%).
Throughout the study, patients with and without BL GCs had a similar mean exposure (SD) to TCZ (1.2 [0.85] vs 1.3 [0.87] years). Still, patients without BL GCs experienced lower rates (events/100 patient years) of adverse events (AEs) and serious AEs (SAEs) considered related to treatment, infections and serious infections (Table 1).
At week 104, 79.9% of the patients with baseline GCs and 15.4% of patients without BL GCs were receiving concomitant GC therapy (Figure 1A). The median GC dose changed by –2.5 mg/d in patient with and by 0.0 mg/d in patients without BL GCs (Figure 1B). Furthermore, the proportion of patients on csDMARDs decreased in both subgroups from BL to week 104 (with GC, 51.2–46.8%; without, GCs 48.4–37.5%).
Similar reductions in mean DAS28-ESR score were observed in patients with and without BL GCs (Figure 2A). CDAI also decreased in both subgroups (Figure 2B), with a mean (SD) reduction of 18.3 (13.3) in patients with and 16.6 (12.6) in those without BL GCs over 104 weeks. Similar reductions in HAQ-DI were also seen in both subgroups during ICHIBAN (Figure 2C).
Conclusion: Even though the majority had low dose GCs only, patients without BL GCs had numerically lower rates of AEs considered related to treatment and infections throughout the ICHIBAN study. Effectiveness endpoints were similar in both subgroups. In patients with BL GCs, the proportion of patients on and dose of GCs decreased throughout the study.
1 Singh et al. Arth Care Res 2015 10.1002/acr.22783
2Specker et al. Clin Exp Rheumatol 2021;39(2):319-328
3Listing et al. Rheumatol 2013;52:53-61
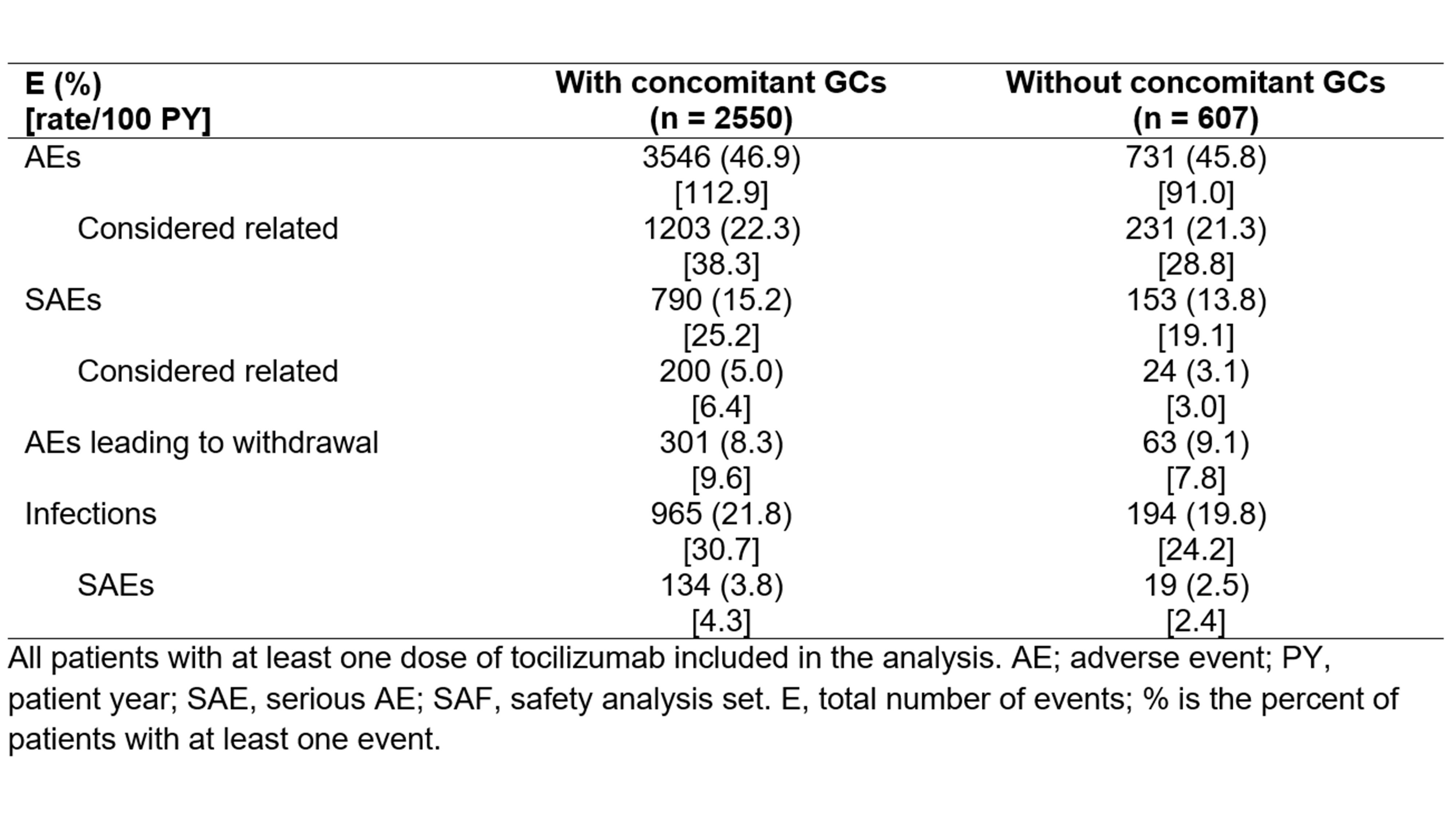 Table 1: Adverse events (AEs) and serious adverse events (SAEs) in patients treated with tocilizumab in subgroups according the concomitant baseline glucocorticoids (GCs).
Table 1: Adverse events (AEs) and serious adverse events (SAEs) in patients treated with tocilizumab in subgroups according the concomitant baseline glucocorticoids (GCs).
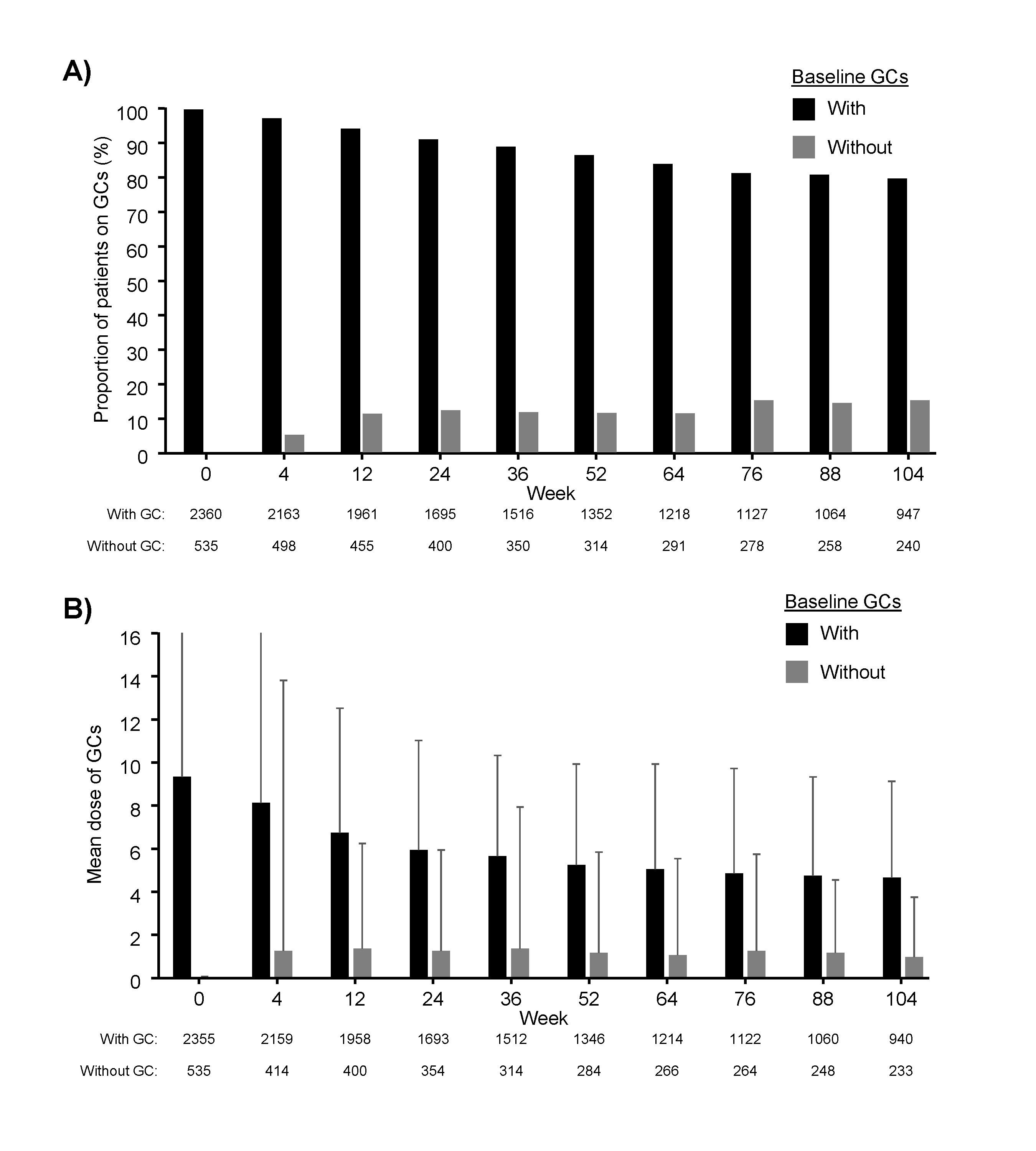 Figure 1: A) The proportion of patients treated with concomintant glucocorticoids (GCs) and B) mean GC dose throughout ICHIBAN by subgroup according to concomitant GCs at baseline. Analysis includes all patients treated with tocilizumab with no prior tocilizumab treatment. Means include patients with no GCs. Error bars represent standard deviation.
Figure 1: A) The proportion of patients treated with concomintant glucocorticoids (GCs) and B) mean GC dose throughout ICHIBAN by subgroup according to concomitant GCs at baseline. Analysis includes all patients treated with tocilizumab with no prior tocilizumab treatment. Means include patients with no GCs. Error bars represent standard deviation.
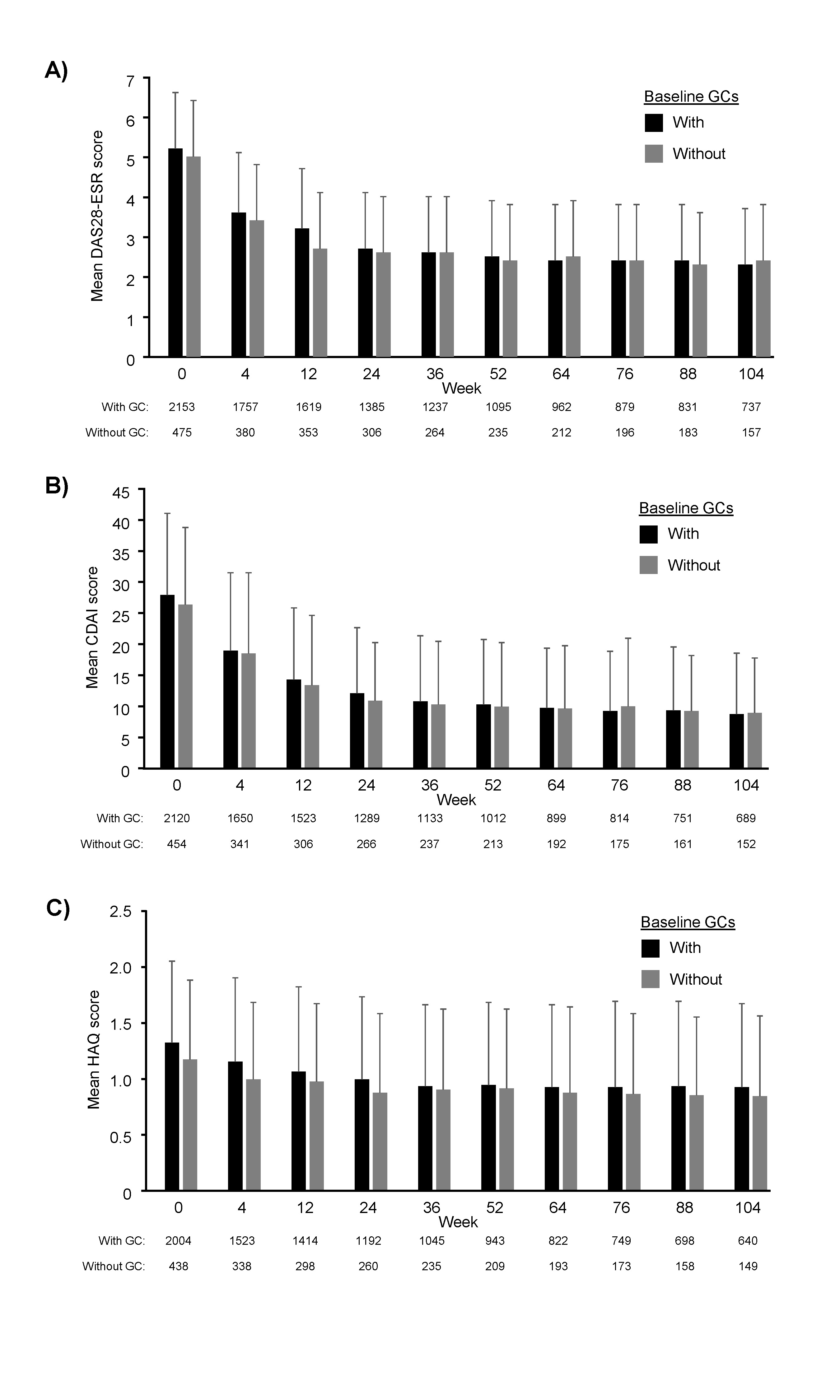 Figure 2: Mean disease activity of patients treated with tocilizumab with or without concomitant baseline glucocorticoids (GCs). A) Mean DAS28-ESR, B) CDAI and C) HAQ-DI scores throughout the ICHIBAN study. Analysis includes all patients treated with tocilizumab with no prior tocilizumab treatment. Error bars represent standard deviation. Missing data was not substituted; CDAI, Clinical Disease Activity Index; DAS28-ESR, Disease Activity Score (DAS) 28 -erythrocyte sedimentation rate, HAQ-DI, Health Assessment Questionnaire – Disability Index.
Figure 2: Mean disease activity of patients treated with tocilizumab with or without concomitant baseline glucocorticoids (GCs). A) Mean DAS28-ESR, B) CDAI and C) HAQ-DI scores throughout the ICHIBAN study. Analysis includes all patients treated with tocilizumab with no prior tocilizumab treatment. Error bars represent standard deviation. Missing data was not substituted; CDAI, Clinical Disease Activity Index; DAS28-ESR, Disease Activity Score (DAS) 28 -erythrocyte sedimentation rate, HAQ-DI, Health Assessment Questionnaire – Disability Index.
To cite this abstract in AMA style:
Specker C, Aringer M, Burmester G, Peters M, Hofmann M, Kellner H, Moosig F, Tony H, Fliedner G. Lower Adverse Event and Infection Rates During Tocilizumab Therapy Without Concomitant GC: An Analysis of the ICHIBAN Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/lower-adverse-event-and-infection-rates-during-tocilizumab-therapy-without-concomitant-gc-an-analysis-of-the-ichiban-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lower-adverse-event-and-infection-rates-during-tocilizumab-therapy-without-concomitant-gc-an-analysis-of-the-ichiban-study/
