Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Traditional induction treatment regimens for proliferative or membranous lupus nephritis (LN) have utilized oral glucocorticoids (GC) in initial doses up to 1.0 mg/kg/day prednisone equivalent with or without a preceding intravenous (IV) methylprednisolone pulse. More recent management guidelines have recommended lower starting oral GC doses following a short IV steroid course, with several clinical trials suggesting this protocol may yield similar results to those using higher GC doses. As there have been no large studies directly comparing responses in patients receiving low vs. high initial oral GC doses for LN treatment, this pooled analysis of randomized controlled trials (RCTs) aims to evaluate differences in efficacy and safety among these groups.
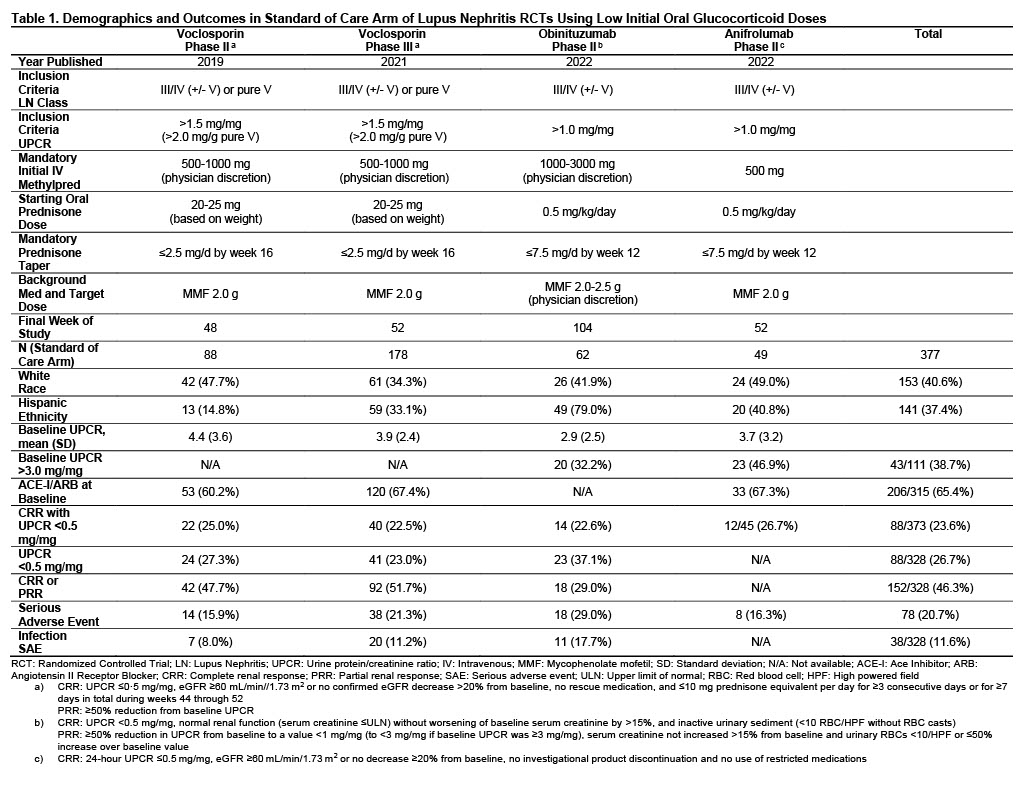
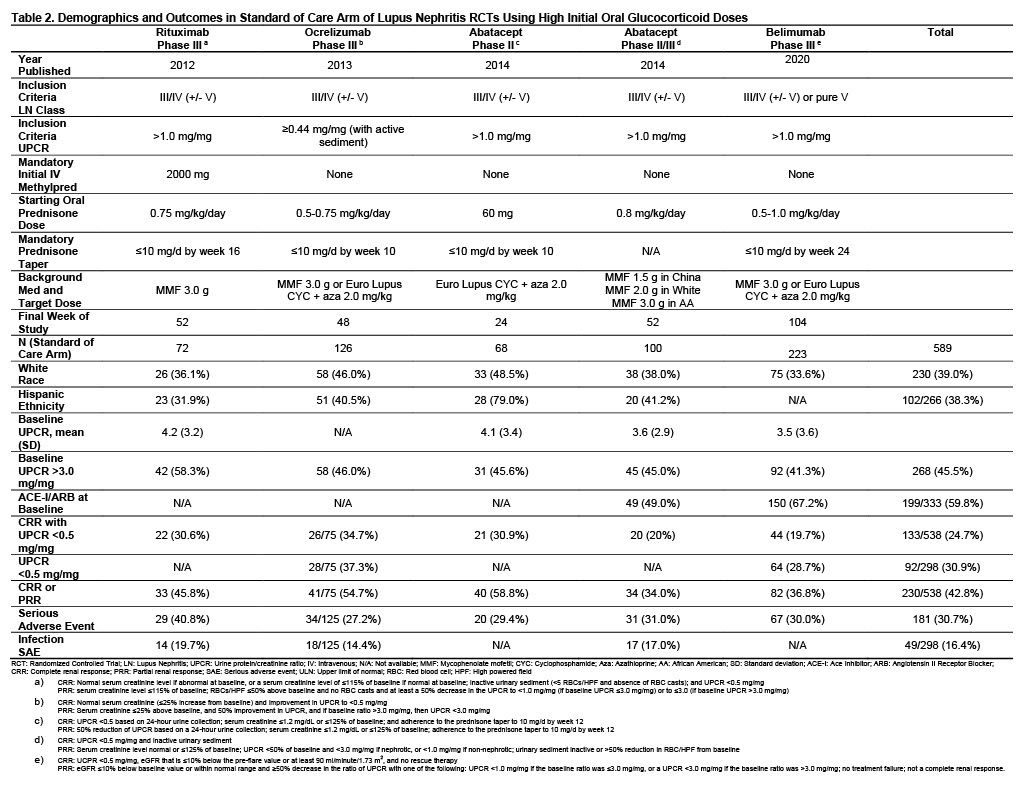
Methods: Published data was analyzed from RCTs that assessed variable doses of GC and an experimental LN treatment with standard of care (SOC) compared to GC and SOC alone. SOC regimens consisted of either mycophenolate mofetil or Euro-Lupus cyclophosphamide followed by azathioprine. Patients from SOC arms receiving a starting prednisone dose of up to 0.5 mg/kg/day (low dose) were compared to those receiving up to 1.0 mg/kg/day (high dose). Complete renal response 0.5 (CRR 0.5) was defined as patients with urine protein/creatinine ratio (UPCR) < 0.5 mg/mg at study completion, along with other parameters that varied between studies (Tables 1, 2). CRR 0.5, serious adverse events (SAE), and where available, isolated UPCR < 0.5 mg/mg, partial renal response (PRR, defined in Tables 1, 2) and SAE due to infections were compared between groups using Fischer’s exact tests.
Results: 377 patients from the SOC arms of 4 studies were exposed to low dose initial GC while 589 patients from 5 studies were treated with high dose GC. LN class and UPCR required for inclusion were generally similar across studies, as were baseline characteristics including white race and, where reported, Hispanic ethnicity, UPCR and percentages on ACE-I/ARB medications (Tables 1, 2). All low dose oral GC studies required an IV steroid pulse compared to only one high dose study. In patients receiving initial low dose oral GC, 23.6% achieved CRR 0.5 at the end of study compared to 24.7% in high dose patients (p=0.75). In reports with available data, similar percentages were seen between groups for isolated UPCR < 0.5 mg/mg (26.7% low dose vs. 30.9% high dose, p=0.29) and CRR or PRR (46.3% low dose vs. 42.8% high dose, p=0.32). SAEs were less common in patients receiving lose dose GC, present in 20.7% compared to 30.7% in high dose patients, p=0.0006. In studies reporting SAE due to infection, low dose GC patients had less frequent events (11.6% vs. 16.4%, p=0.08).
Conclusion: Based on data pooled from multiple published trials utilizing SOC treatment for LN, there is no significant difference in renal responses between patients receiving IV steroid followed by low dose prednisone compared to those receiving high prednisone doses as initial oral GC during induction. Serious adverse events were less frequent in patients receiving low dose initial GC, although other study-specific considerations may factor in that association. These findings support the use of lower oral GC doses in LN treatment.
To cite this abstract in AMA style:
Saxena A, Izmirly P, Law J, Sorrento C, Belmont H, Buyon J. Low vs. High Initial Oral Glucocorticoid Dose for Lupus Nephritis Induction Treatment: A Pooled Analysis of Randomized Controlled Clinical Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/low-vs-high-initial-oral-glucocorticoid-dose-for-lupus-nephritis-induction-treatment-a-pooled-analysis-of-randomized-controlled-clinical-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-vs-high-initial-oral-glucocorticoid-dose-for-lupus-nephritis-induction-treatment-a-pooled-analysis-of-randomized-controlled-clinical-trials/