Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Low-dose Interleukin-2 (Ld-IL2) has shown therapeutic effect in autoimmune diseases, particularly systemic lupus erythematosus (SLE). Various doses from 0.33 to 3.0 million units of IL-2 have been used widely in clinics. As far, the optimal dose in SLE treatment has not been evaluated, which leads to uncertainty of efficacy and causes difficulty of dose decision in practice.
Methods: In this multicenter, randomized, double-blind, Phase IIb trial, 152 active SLE patients were randomized (1:1:1:1) to receive subcutaneous IL2 (0.2, 0.5, or 1.0 million IU) or placebo every other day for 12 weeks, then weekly for further 12 weeks.
Results:
At week 12, significantly higher SRI-4 response rates were demonstrated in IL2 1M IU (69.7%), 0.5M IU (64.7%), and 0.2M IU (42.9%) than in placebo (23.5%). Notably, these significant differences proceeded until the end of treatment period at week 24 (p < 0.001). Secondary outcomes revealed SLE patients achieving LLDAS at doses of 1M IU, 0.5M, and 0.2M (51.5%, 37.1%, and 28.5% respectively) at week 24. In the 1M IU Ld-IL2 group, significant reductions were observed in PGA scores, anti-dsDNA antibody, and prednisone dosages, accompanied by remarkable increases in serum C3 and C4 levels. Infection rates were lower in IL2 groups compared to placebo. Ld-IL2 drives expansion of Tregs and rebalance of Tregs/Teff ratio.
Conclusion: Ld-IL2 demonstrates efficacy for patients with SLE in a dose-dependent manner, associated with expansion of Tregs and rebalance of Tregs/Teff ratio.
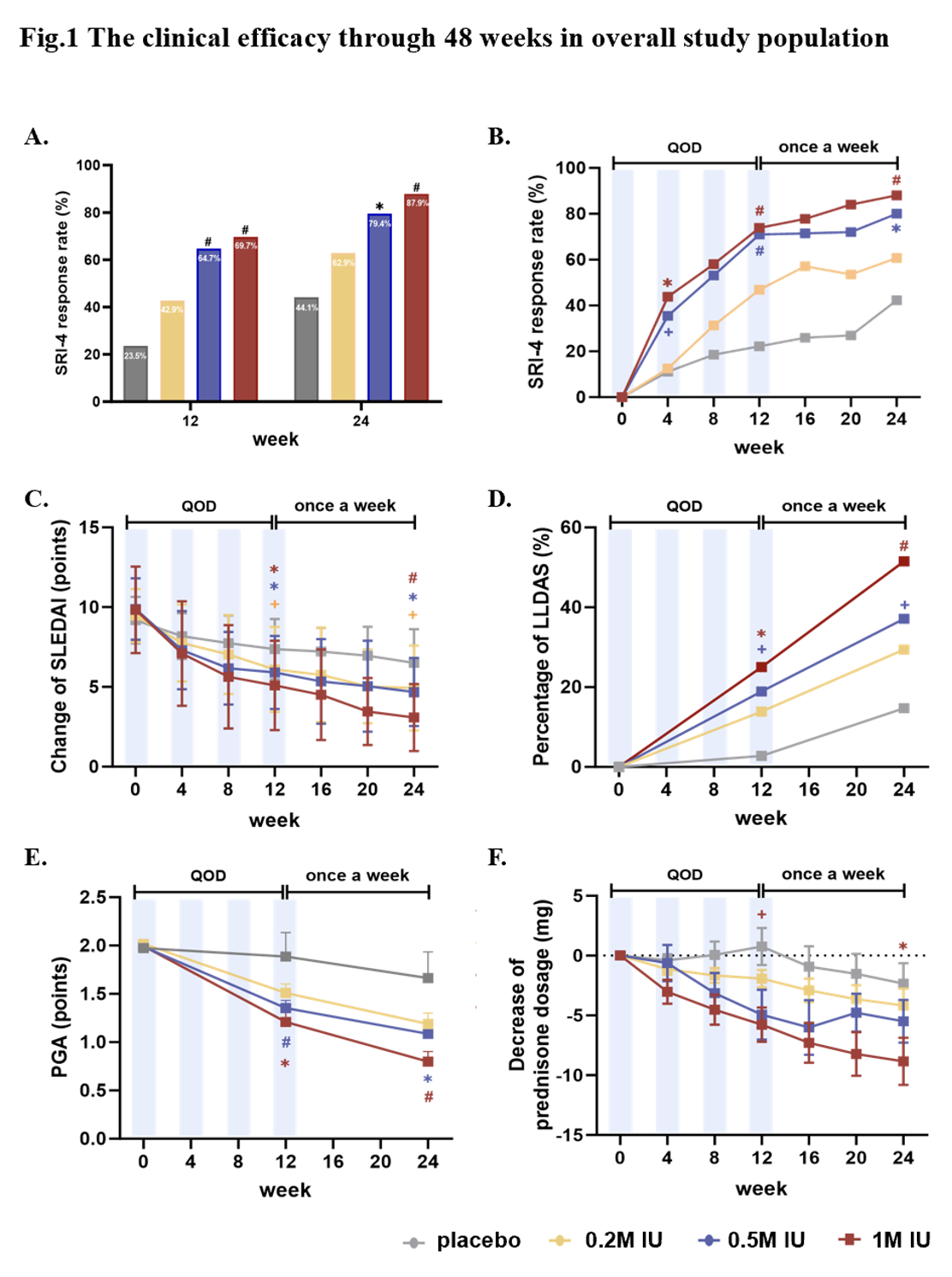 Figure 1. The clinical efficacy through 48 weeks in overall study population
Figure 1. The clinical efficacy through 48 weeks in overall study population
(A) Efficacy of Ld-IL2 assessed by SRI-4 during 24 weeks. (B) Percentage of SRI-4 responders at week 12 and 24. (C) Change of SLEDAI from baseline to 24 weeks. (D) Proportion of LLDAS responders at week 12 and 24. (E) Improvement with Ld-IL2 determined by PGA. (F) Change of prednisone dose from baseline to week 24.
Red represents Ld-IL2 1M IU versus placebo group; blue Ld-IL2 0.5M IU versus placebo group; yellow represents Ld-IL2 0.2M IU versus placebo group.
Ld-IL-2, low-dose interleukin 2. SRI-4: Systemic Lupus Erythematosus Responder Index 4; PGA: physician‘s global assessment. + P<0.05; *P<0.01; # P<0.001
.jpg?2) Figure 2. Dynamics of laboratory and immunological responses after IL2 treatment
Figure 2. Dynamics of laboratory and immunological responses after IL2 treatment
(A-B) Change in C3 and C4 concentrations from baseline over time. (C-F) Changes of the percentage and the number of foxp3+ CD127loCD25hi regulatory T cells (foxp3+Treg) and effector T (Teff) cells.
Red represents Ld-IL2 1M IU versus placebo group; blue Ld-IL2 0.5M IU versus placebo group; yellow represents Ld-IL2 0.2M IU versus placebo group.
Ld-IL-2, low-dose interleukin 2. SRI-4: Systemic Lupus Erythematosus Responder Index 4; PGA: physician’s global assessment. + P<0.05; *P<0.01; # P<0.001
To cite this abstract in AMA style:
Zhang X, Feng R, Li Z, He J. Low-dose Interleukin-2 Therapy in Systemic Lupus Erythematosus: a double-blind, randomised, placebo-controlled, phase IIb trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/low-dose-interleukin-2-therapy-in-systemic-lupus-erythematosus-a-double-blind-randomised-placebo-controlled-phase-iib-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-dose-interleukin-2-therapy-in-systemic-lupus-erythematosus-a-double-blind-randomised-placebo-controlled-phase-iib-trial/
