Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Renal flares contribute substantially to morbidity, renal survival and death in systemic lupus erythematosus (SLE). Identification of pharmacological strategies for the prevention of renal flares is a key unmet need in SLE treatment. In the present study, we tested the hypothesis that the use of belimumab and antimalarial agents (AMA) protects against the development of renal flares.
Methods: We pooled data from the BLISS-52, BLISS-76, BLISS-SC and BLISS-Northeast Asia (NEA) randomised clinical trials of belimumab (N=3225). Serologically active SLE patients with active disease were recruited and followed for 52 weeks in BLISS-52, BLISS-SC and BLISS-NEA and for 76 weeks in BLISS-76; patients with active severe lupus nephritis (LN) were excluded. Patients were allocated to receive intravenous (IV) belimumab 1 mg/kg (N=559), IV belimumab 10 mg/kg (N=1033), SC belimumab 200 mg (N=556) or placebo (N=1077) in addition to standard therapy. The outcome of the present post-hoc analysis was development of renal flares, defined as a reproducible (i) increase in proteinuria to >1 g/day if the baseline value was < 0.2 g/d; >2 g/day if the baseline value was 0.2–1.0 g/d; or >2 times the baseline value if this was >1g/d, (ii) increase in serum creatinine ≥20% or 0.3 mg/dL, accompanied by proteinuria, haematuria or red blood cell (RBC) casts, or (iii) new haematuria of glomerular origin, accompanied by proteinuria or RBC casts. The hazard of renal flare was assessed with Cox proportional hazards regression models. Analyses were adjusted for age, sex, ethnicity, previous renal involvement, baseline proteinuria and glomerular filtration rate, and use of glucocorticoids and immunosuppressants.
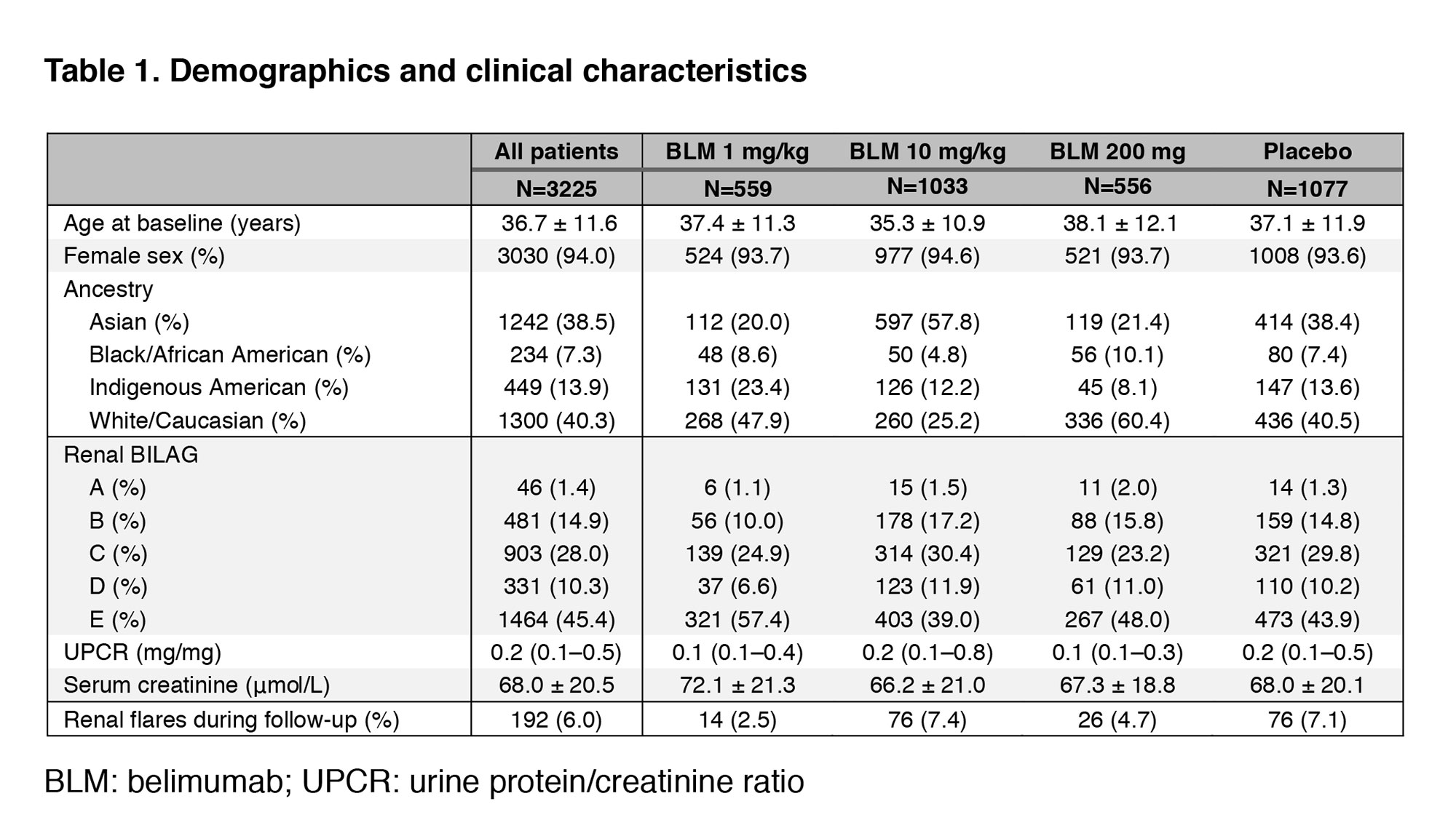
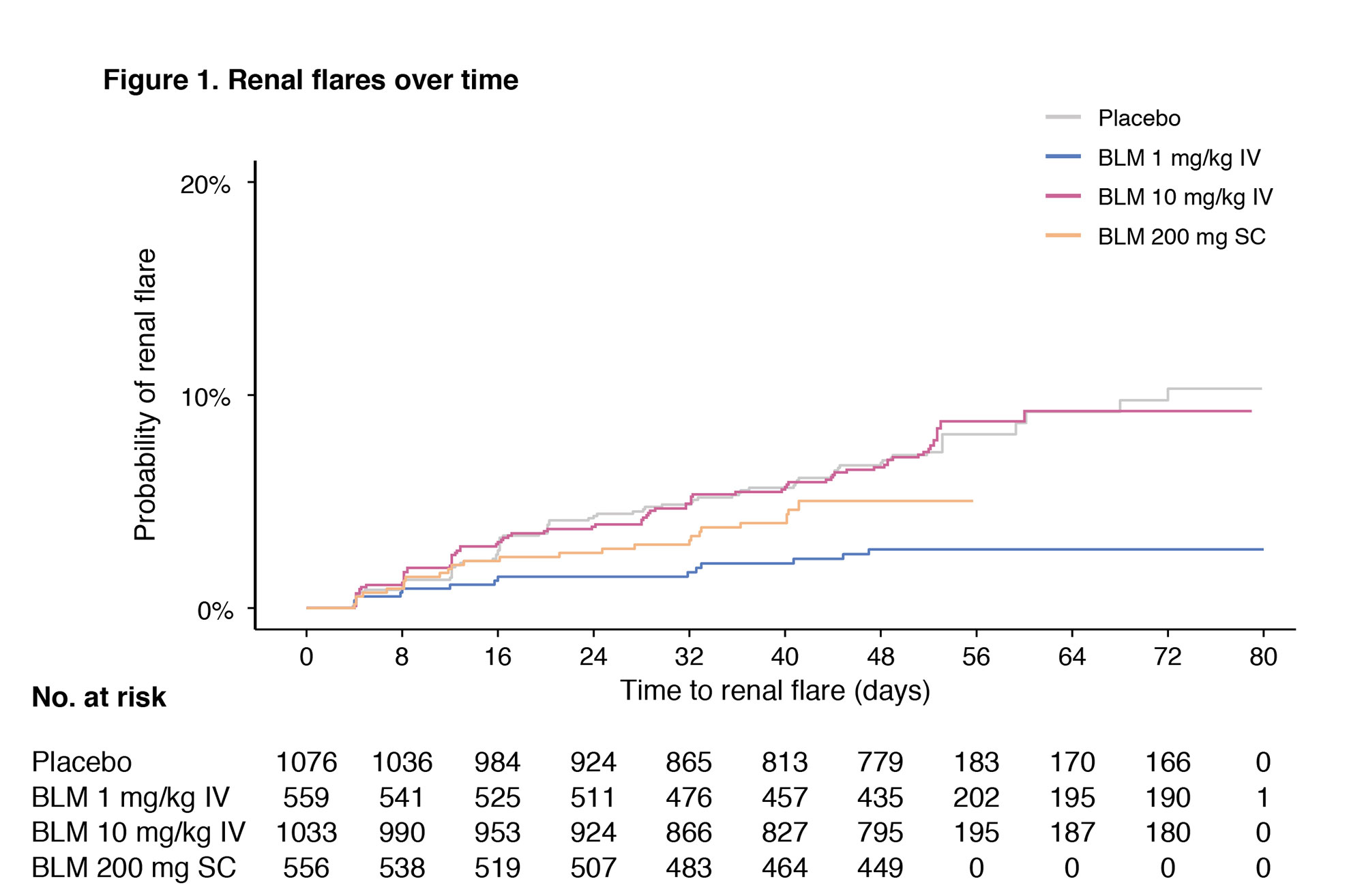
Results: Demographic and clinical characteristics are shown in Table 1. The proportion of patients presenting a renal BILAG A–D at baseline was 54.6%. In the pooled population, 192 patients developed a renal flare after a median of 141 days. In multivariable Cox regression analysis, use of AMA was associated with a lower risk of renal flares (hazard ratio [HR]: 0.66; 95% confidence interval [CI]: 0.49–0.88; p=0.005). Compared with placebo, the risk of renal flares was lower among patients receiving IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.25–0.81; p=0.007), but not IV belimumab 10 mg/kg (HR: 0.79; 95% CI: 0.57–1.09; p=0.148) or SC belimumab 200 mg (HR: 0.93; 95% CI: 0.58–1.47; p=0.749). In subgroup analyses including patients with renal BILAG A–D at baseline, these negative associations with renal flares held true for the use of AMA (HR: 0.67; 95% CI: 0.49–0.92; p=0.013) and IV belimumab 1 mg/kg (HR: 0.45; 95% CI: 0.24–0.87; p=0.017).
Conclusion: Low-dose belimumab and AMA were independently associated with a lower risk of renal flare in patients with active SLE from a clinical trial setting. Add-on high-dose belimumab has been approved for the treatment of active LN; the inability to show a protective effect of high-dose belimumab against renal flares in the present study implies that proteinuria levels may have to be considered for dose-adjustment of belimumab in clinical practice, and that investigation of the effect of intermediate doses of belimumab bears merit.
To cite this abstract in AMA style:
Gomez A, Jägerback S, Sjöwall C, Parodis I. Low-dose Belimumab and Antimalarial Agents Prevent Renal Flares in Systemic Lupus Erythematosus: Results from Four Randomised Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/low-dose-belimumab-and-antimalarial-agents-prevent-renal-flares-in-systemic-lupus-erythematosus-results-from-four-randomised-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-dose-belimumab-and-antimalarial-agents-prevent-renal-flares-in-systemic-lupus-erythematosus-results-from-four-randomised-clinical-trials/