Session Information
Date: Sunday, November 10, 2019
Title: 3S105: Osteoarthritis & Joint Biology – Basic Science (886–891)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
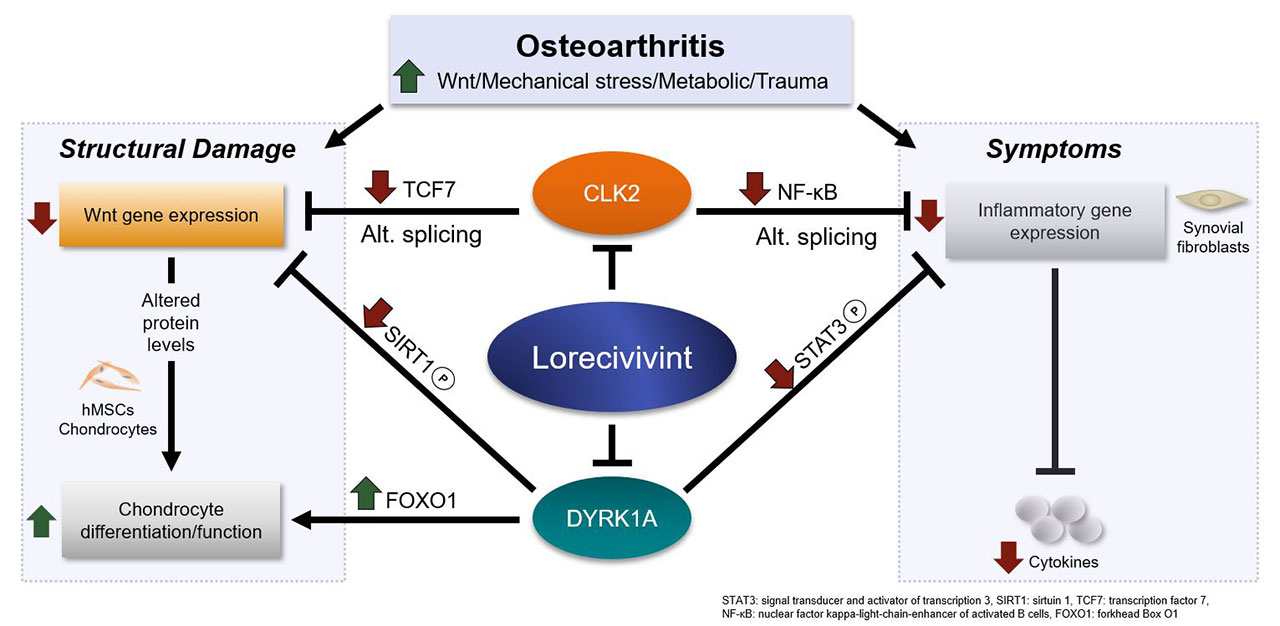
Background/Purpose: In the synovial joint, Wnt pathway upregulation contributes to osteoarthritis (OA) by increasing osteocyte differentiation, cartilage thinning, and inflammation. Lorecivivint (LOR; SM04690), a novel small molecule, has previously demonstrated potential OA disease-modifying effects through Wnt pathway inhibition in vitro and in vivo. These studies sought to elucidate the novel mechanism of action for LOR on Wnt pathway inhibition, chondrocyte differentiation, and anti-inflammation.
Methods: Wnt pathway activity was measured using a cell-based TCF/LEF luciferase reporter in SW480 colon cancer cells. A kinome screen (318 kinases) was performed. The effects of LOR on protein phosphorylation of serine and arginine rich splicing factors (SRSF proteins), Sirt1, and FoxO1 in hMSCs, chondrocytes, and synovial fibroblasts were measured by Western blot. The effects of LOR and siRNA knockdown (KD) on chondrogenic and Wnt pathway gene expression were measured by NanoString gene expression panels and effects on LPS-induced inflammatory cytokines (IL-6, IL-8, TNF-α) in BEAS-2B cells were measured by qPCR and ELISA. In vivo, the pharmacodynamic effects of LOR were evaluated in monosodium iodoacetate injection-induced and anterior cruciate ligament transection with partial medial meniscectomy rat knee OA models in which a single intra-articular LOR (0.1 µg, 0.3 µg, 1.0 µg) or vehicle injection was administered. Cartilage was isolated at Day 10 and 35; phosphorylation and expression of SRSF proteins, Sirt1, FoxO1, STAT3, and NF-κB were measured by Western blot.
Results: LOR was a potent (EC50=11nM) inhibitor of Wnt signaling. CDC-like kinases (CLKs) and dual-specificity tyrosine kinase (DYRK1A) were identified as molecular targets of LOR. In hMSCs and chondrocytes, compared to DMSO, LOR potently inhibited CLK-mediated phosphorylation of SRSF proteins. LOR also inhibited DYRK1A-mediated Sirt1 and FoxO1 phosphorylation, thus increasing total and nuclear FoxO1 levels. Compared to siRNA control, DYRK1A/CLK2 dual KD increased expression of chondrogenic genes (COL2A1, ACAN, COMP, CD44 [all P< 0.05]). Compared to siRNA control, CLK2 and DYRK1A KDs each inhibited Wnt pathway genes (AXIN2, TCF7, TCF4, LRP5, FZD6, FZD7, PITX2 [all P< 0.05]) with no effects on β-catenin levels. In synovial fibroblasts, compared to DMSO, LOR decreased phosphorylation of NF-kB and STAT3. In BEAS-2B cells, compared to siRNA control, DYRK1A KD inhibited inflammatory cytokine production (IL-6, IL-8, TNF-α [all P< 0.05]) while DYRK1A/CLK2 dual KD enhanced anti-inflammatory effects of DYRK1A KD. In cartilage from rat OA models, compared to vehicle, LOR inhibited phosphorylation of SRSF proteins, Sirt1, FoxO1, and STAT3 as well as expression of NF-κB.
Conclusion: Inhibition of nuclear kinases CLK2 and DYRK1A leads to effects on the Wnt pathway, chondrocytes, and inflammation (Image). This dual mechanism of LOR potentially modifies OA through increased chondrocyte differentiation and function and benefits symptoms through anti-inflammatory activity. Human trials are ongoing.
To cite this abstract in AMA style:
Deshmukh V, O'Green A, Bossard C, Seo T, Lamangan L, Ibanez M, Ghias A, Lai C, Do L, Cho S, Cahiwat J, Chiu K, Pedraza M, Yazici Y. Lorecivivint (SM04690), a Potential Disease-Modifying Osteoarthritis Drug, Inhibits CLK2 and DYRK1A, Novel Molecular Regulators of Wnt Signaling, Chondrogenesis, and Inflammation [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/lorecivivint-sm04690-a-potential-disease-modifying-osteoarthritis-drug-inhibits-clk2-and-dyrk1a-novel-molecular-regulators-of-wnt-signaling-chondrogenesis-and-inflammation/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lorecivivint-sm04690-a-potential-disease-modifying-osteoarthritis-drug-inhibits-clk2-and-dyrk1a-novel-molecular-regulators-of-wnt-signaling-chondrogenesis-and-inflammation/