Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous studies have noted significant variation in serum urate (sUA) levels, and it is unknown how this influences the accuracy of hyperuricemia classification based on single data points. Despite this known variability, hyperuricemic patients are often used as a control group in gout studies. Our objective was to determine the accuracy of hyperuricemia classifications based on single data points versus multiple data points given the degree of variability observed with serial measurements of sUA.
Methods: Data was analyzed from 85 young adults without a gout diagnosis participating in a single center, double-blinded, crossover trial in which participants were randomly assigned to receive allopurinol (300 mg daily) or placebo for a period of 4 weeks. Serum urate levels were measured at five clinic encounters (2 -4 week intervals between measurements). For this analysis, sUA levels collected at screening, post-placebo and post-washout (i.e., no intervention) were considered (up to 4 sUA levels per participant). Mean coefficient of variation for sUA was determined. The rates of conversion from normouricemia (sUA ≤6.8 mg/dL) to hyperuricemia (sUA >6.8 mg/dL), and from hyperuricemia to normouricemia at were calculated. The rates of conversion to hyperuricemia were then compared across subgroups defined by the sUA level at initial screening (4-4.4, 4.5-4.9, 5-5.4, 5.5-5.9, 6-6.8).
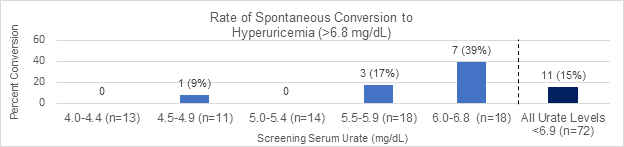
Results: Mean study participant (n = 85) age was 27.8±7.0 years and mean body mass index was 31.1±7.9. 39% of participants were women. 41% of participants were African-American. Mean sUA coefficient of variation was 8.5% ± 4.9% (1% to 23%). There was no significant difference in the coefficient of variation between men and women, or between participants whose screening values were normouricemic (sUA ≤6.8 mg/dL) and those whose values were hyperuricemic (sUA >6.8 mg/dL). Among those with an initial sUA value in the range of normouricemia (n=72), 15% converted to hyperuricemic levels during at least one subsequent measurement (figure 1). The subgroup with initial sUA < 6.0 (n=54) was much less likely to have future values in the range of hyperuricemia compared to the group with screening sUA values between 6.0-6.8 (n=18) (20% vs 39%, p = 0.0037). Of the study participants with a screening sUA value in the range of hyperuricemia (n=13), 46% had values in normouricemia ranges during at least one later measurement.
Conclusion: Single sUA measurements were unreliable in hyperuricemia categorization (defined as sUA >6.8 mg/dL) due to spontaneous variation in urate levels. This is likely a result of multiple factors such as time of sample collection, diet, and change in weight. Those with an sUA measurement of < 6.0 mg/dL were less likely to demonstrate sUA values classified as hyperuricemic at future evaluations, and this could be considered a safer threshold to rule out intermittent hyperuricemia based on a single measurement point.
To cite this abstract in AMA style:
Shaffer A, Rahn E, Saag K, Mudano A, Gaffo A. Longitudinal Variation in Repeat Serum Urate Levels: Relationship with Hyperuricemia Classification [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/longitudinal-variation-in-repeat-serum-urate-levels-relationship-with-hyperuricemia-classification/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-variation-in-repeat-serum-urate-levels-relationship-with-hyperuricemia-classification/