Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: T cells are critical for control of viral infection with SARS-CoV-2, but knowledge is lacking on cellular immune responses following repeated vaccination and breakthrough infection in immunosuppressed patients. The objective was to examine longitudinal T cell responses across diagnoses, following vaccine series and COVID-19 in patients on tumor necrosis factor inhibitors (TNFi).
Methods: The prospective, observational Nor-vaC study included patients with arthritis (spondyloarthritis, rheumatoid arthritis, psoriatic arthritis) or inflammatory bowel disease (IBD) (ulcerative colitis, Crohns disease) on immunosuppressive therapies. Here, we included patients on TNFi mono- or combination therapy immunised with up to four SARS-CoV-2 vaccine doses with or without breakthrough infection, collecting peripheral blood mononuclear cells (PBMCs) 2-4 weeks after each immunisation. Samples were incubated with SARS-CoV-2 spike, nucleocapside or membrane peptides. The percentage of responding T cells was measured by flow cytometry.
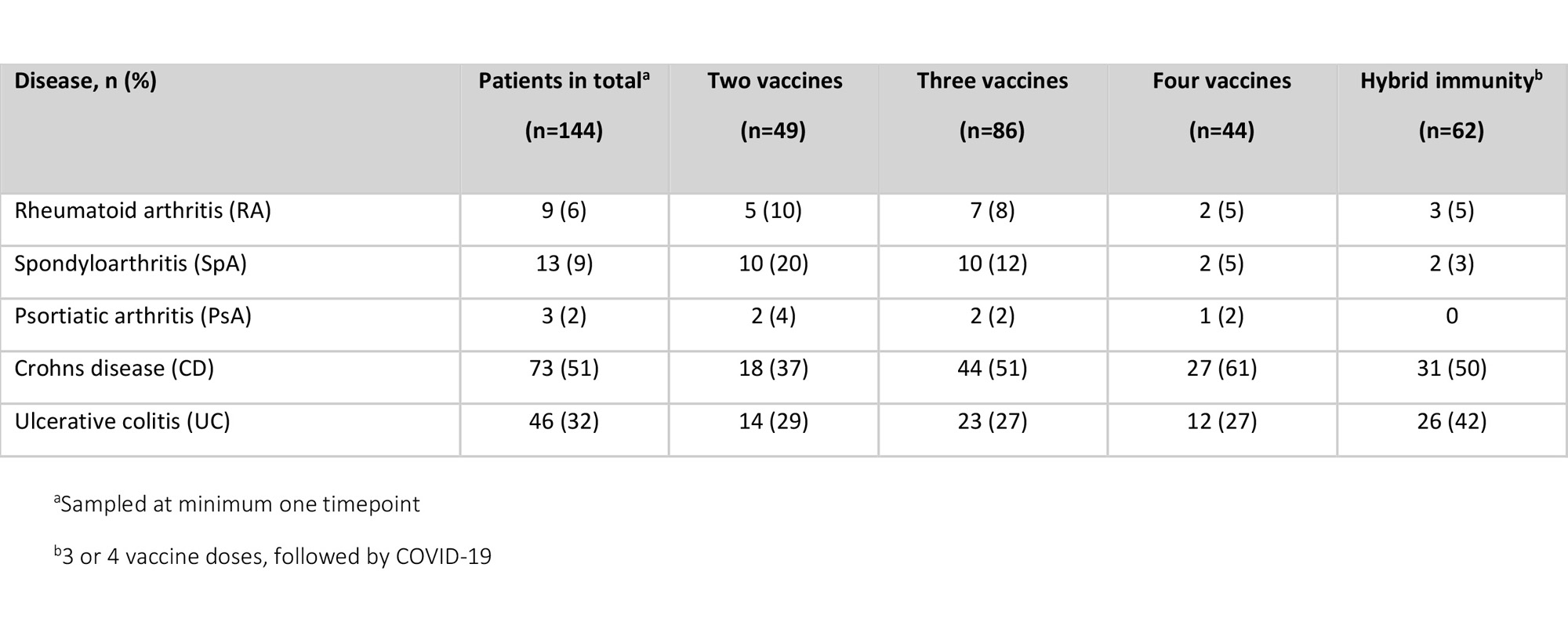
Results: Between February 2021 and December 2022, 144 patients on TNFi (monotherapy n=86 (60%), combination with methotrexate or azathioprine n=58 (40%)) were included (median age 48 years [IQR 33-57]; 51% women) (Table).
The proportions of arthritis vs IBD patients with CD4 responses after 2 vaccine doses were 75% (12/16 patients) vs 86% (25/29), and after 3 doses 83% (10/12) vs 93% (28/30). In total, 80% (4/5) of arthritis patients showed further increases in CD4 responses after a 4th vaccine dose.
Conversely, 81% (13/16) of arthritis patients vs. 55% (16/29) of IBD patients had CD8 T cell responses after two doses, and 67% (8/12) vs. 62% (18/29) after three doses. A 3rd and 4th dose induced higher CD8 responses compared to the previous dose in 55% (6/11) and 100% (5/5) of arthritis patients.
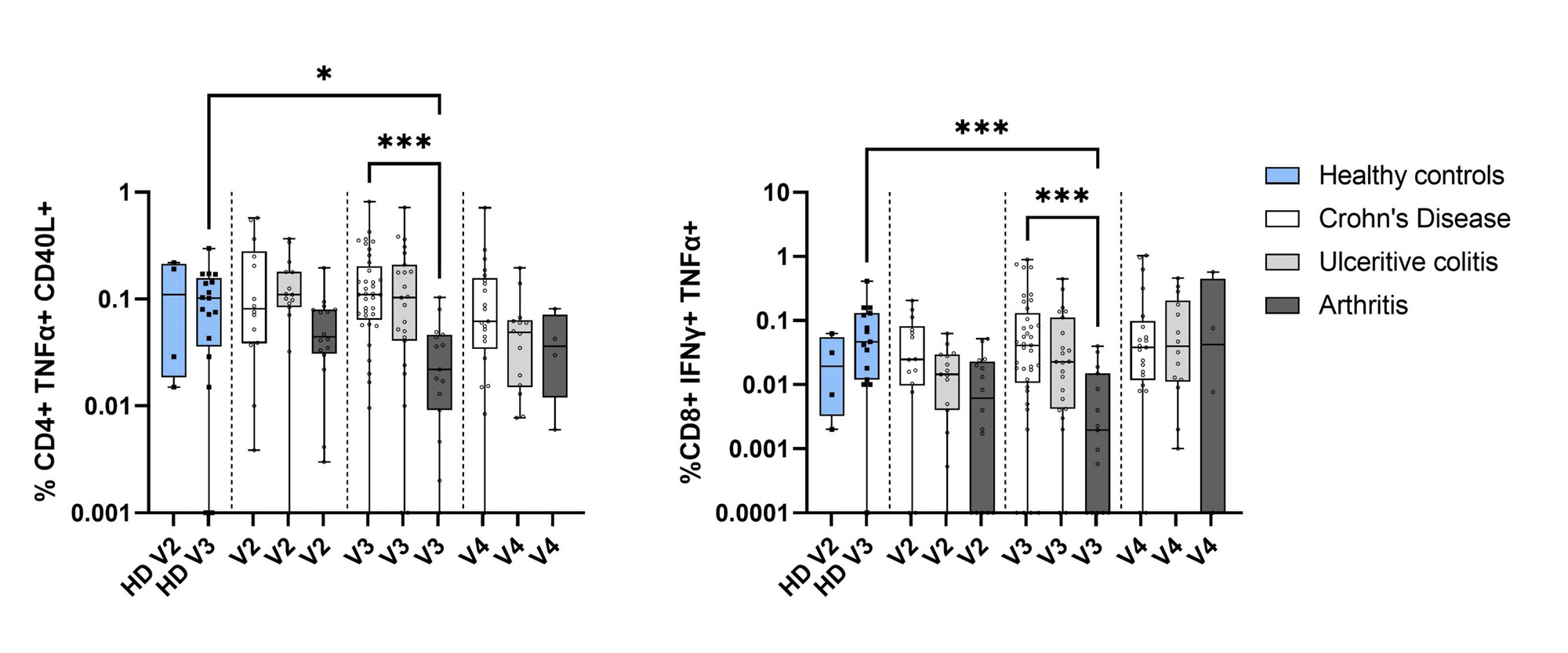
Arthritis patients had lower T cell responses than IBD patients after the 3rd dose; median CD4 response 0.024% [IQR 0.009-0.036] vs 0.098% [IQR 0.040-0.182], p=0.0004; median CD8 response 0.003% [IQR 0.001-0.016] vs 0.044% [IQR 0.009-0.140], p=0.0032 (Figure). This difference remained robust after adjusting for age and sex, p< 0.001, but was no longer detected after the 4th vaccine dose.
Breakthrough infection elicited increased T cell responses across all diagnoses to spike (p< 0.0001), and to nucleocapsid (p=0.002) and membrane proteins (p=0.001) compared to unstimulated T cells.
Also, Spike-specific T cell responses increased compared to the 3rd dose (median CD4 T cell response 0.18% vs. 0.06%, p=0.003; CD8 T cell response 0.08% vs. 0.01%, p< 0.0001), but to a lesser extent compared to the 4th dose (median CD4 response 0.18% vs. 0.12%, p=0.05; CD8 response 0.08% vs. 0.05%, p=0.26).
Conclusion: Patients on TNFi show improved cellular responses following each immunisation, with infection generating a strong and broad T cell response. Arthritis patients had significantly lower cellular responses compared to IBD patients after three vaccine doses, but with no difference after the 4th dose. These results support giving a 4th vaccine dose to TNFi-treated patients, with particular benefit for arthritis patients.
To cite this abstract in AMA style:
Ørbo H, Wolf A, Bjørlykke K, Josefsson S, Solum G, Kjønstad I, Jyssum I, Christensen I, Tveter A, Sexton J, Kro G, Grødeland G, Kvien T, Jahnsen J, Vaage J, Haavardsholm E, Provan S, Kared H, Munthe L, Jørgensen K, Syversen S, Mjaaland S, Goll G. Longitudinal T Cell Responses to a Series of Four SARS-CoV-2 Vaccine Doses or COVID-19 in Patients on TNF Inhibitors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/longitudinal-t-cell-responses-to-a-series-of-four-sars-cov-2-vaccine-doses-or-covid-19-in-patients-on-tnf-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-t-cell-responses-to-a-series-of-four-sars-cov-2-vaccine-doses-or-covid-19-in-patients-on-tnf-inhibitors/