Session Information
Date: Sunday, October 26, 2025
Title: Plenary I (0772–0776)
Session Type: Plenary Session
Session Time: 10:15AM-10:30AM
Background/Purpose: Seropositive rheumatoid arthritis (RA) has an at-risk stage identifiable by elevations of antibodies to cyclic citrullinated peptide (CCP). Identifying the immunologic factors that distinguish CCP(+) at-risk individuals who progress to RA (Converters) from those who do not (Nonconverters) is crucial to develop strategies for prediction and prevention of clinical RA.
Methods: We used longitudinal data from CCP3(+) individuals in the StopRA trial (NCT02603146) that found hydroxychloroquine (HCQ) was not effective in preventing clinical RA. We used single-cell multi-omics on peripheral blood mononuclear cells from pre-RA/baseline and incident RA-like synovitis timepoints in Converters and at baseline and follow-up in Nonconverters (Fig 1A-C). For T cell analysis, we integrated CITE-seq and mass cytometry (CyTOF) data to identify shared T cell clusters, confirming concordant expression patterns across technologies (Fig 1D-E). We also developed a composite predictive model for clinical RA (Fig 1F).
Results: 47 Converters and 53 Nonconverters underwent CyTOF analyses, and 24 Converters and 12 Nonconverters iCITE-seq/scASAP-seq. At baseline (V0), T peripheral helper (Tph) cells (T-14) were significantly elevated in Converters vs Nonconverters; Converters also had expanded GZMK+XCL1+CD57+ CD8+ T cells (Fig 2A-B). Differential gene expression analysis also showed that at baseline Converters had upregulation of cytotoxic genes (e.g., GZMB) in CD8+ T cells, Th2-associated genes (e.g., CCR4) and TNF in CD4+ T cells, among others, indicating broad dysregulation of inflammatory and cytotoxic pathways (Fig 2C-D). Single-cell chromatin accessibility profiling showed that specific chromatin regions were accessible at baseline in Converters, particularly in immune regulatory loci (Fig 2E-F). Notably, the PTPN22 locus exhibited enhanced chromatin accessibility in NK cells at baseline in Converters (Fig 2G-I). In longitudinal analyses, TCR analysis showed expanded clones dominated by Tph cells and GZMB+ and GZMK+ CD8+ T cells in Converters at baseline and at synovitis onset (V1) (Fig 2J-M). A predictive model using baseline Tph frequency, CCP3 levels, and rheumatoid factor (RF) distinguished Converters from Nonconverters with an AUC of 0.771 (Fig 3A). A decision tree model further identified those with CCP3 ≥108 units and Tph ≥2.45% of CD3+ T cells had the highest risk for conversion (Fig 3B). Kaplan-Meier demonstrated that, at baseline, Converters in the top 20% for Tph or CCP3 levels had a significantly shorter time to RA onset compared to the bottom 20% (Fig 3C-D).
Conclusion: Alterations in cellular subsets, gene expression, chromatin accessibility and clonal expansion are present in at-risk individuals with CCP3 elevations who develop synovitis. These findings inform potential targets for preventive interventions in clinical trials as well as prediction models to identify CCP3(+) individuals at highest risk for developing clinical RA. Notably, while HCQ did not show clinical effect, future studies will evaluate HCQ effect on cells and other biomarkers.
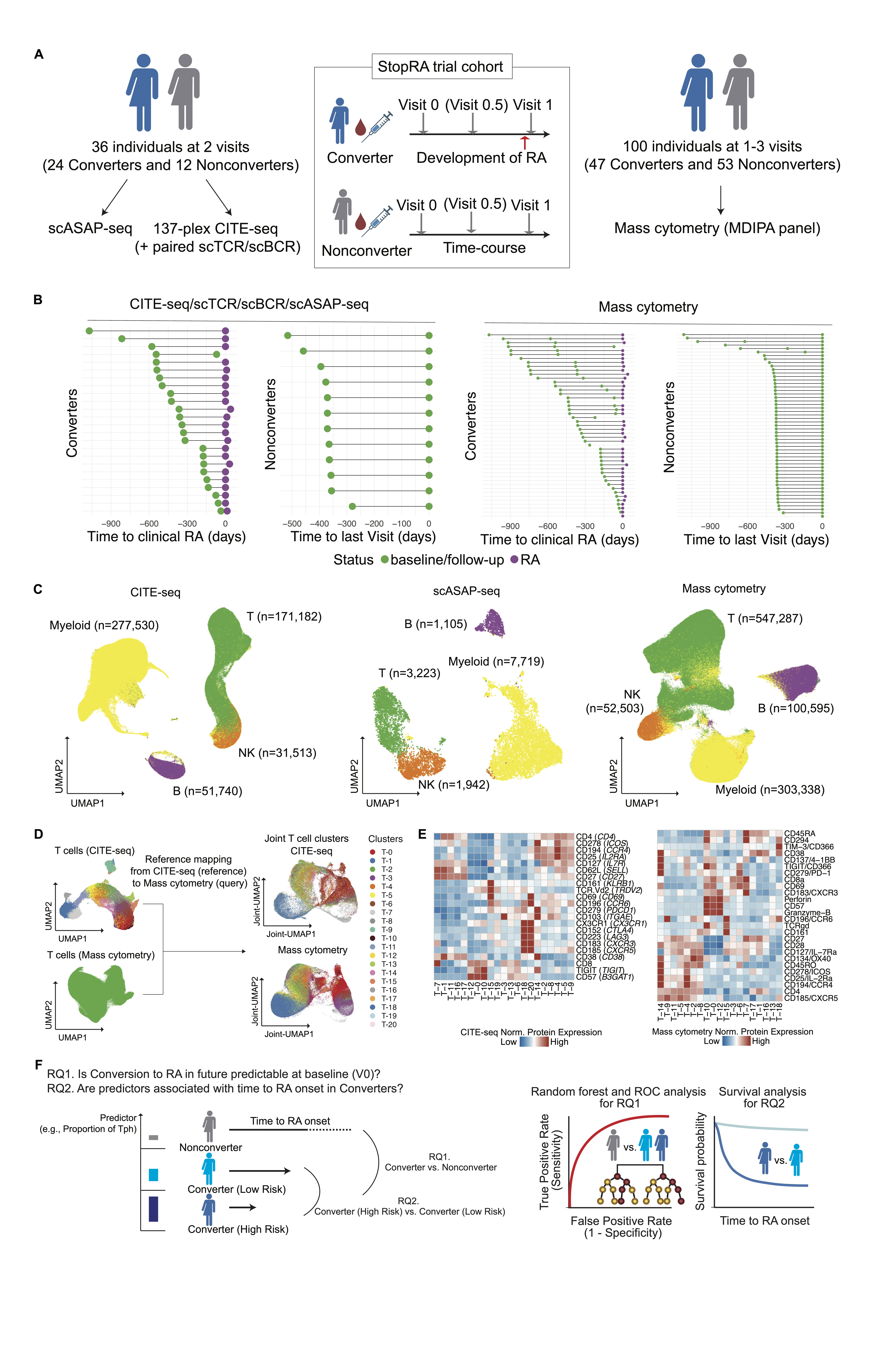 Fig.1: Study design for longitudinal single-cell omic data collection from RA-Converters and Nonconverters. A, The study cohort was selected from 144 individuals with anti-CCP3 levels >=2 times the upper limit of normal who were enrolled and followed longitudinally in the StopRA trial that evaluated the efficacy of hydroxychloroquine (HCQ) in a placebo-controlled fashion to prevent clinical RA. A subset of 36 individuals (24 Converters and 12 Non-Converters) was profiled using single-cell ASAP-seq and 137-plex CITE-seq, including paired single-cell TCR and BCR sequencing. Another subset of 100 individuals (47 Converters and 53 Non-Converters) was analyzed with mass cytometry (MDIPA panel) across 1–3 visits. B, Time points of sample collection per individual. The time to clinical RA diagnosis (day 0) is shown for Converters, and a follow-up visit is indicated for Nonconverters. Data are presented separately for individuals analyzed by CITE-seq/scTCR-seq/scBCR-seq/scASAP-seq (left) and those analyzed by mass cytometry (right). Colored points represent visit status: baseline/follow-up (green) or post-RA (purple). C, Broad immune cell-type identification presented in the UMAP, showing major immune cell types identified across CITE-seq, scASAP-seq, and mass cytometry datasets. D, Strategy to integrate CITE-seq and mass cytometry data for T cell analysis. UMAP projection of integrative T cell subclusters projected into a shared embedding space using overlapping measured 33 proteins with CITE-seq data as reference and mass cytometry as query. Clusters in CITE-seq and joint-space are color-coded and labeled consistently across modalities. E, Normalized protein expression in joint T cell subclusters from CITE-seq (left) and mass cytometry data (right). F, Schematic of the predictive modeling approach. Two research questions were addressed: (RQ1) distinguishing Converters from Nonconverters at baseline (V0) and (RQ2) predicting time to RA onset among Converters. Predictors such as Tph proportions were incorporated into random forest model to prioritize them. Note: CyTOF analyses included study participants who received HCQ or placebo, while CITE-seq and ASAP-seq only included participants who received placebo.
Fig.1: Study design for longitudinal single-cell omic data collection from RA-Converters and Nonconverters. A, The study cohort was selected from 144 individuals with anti-CCP3 levels >=2 times the upper limit of normal who were enrolled and followed longitudinally in the StopRA trial that evaluated the efficacy of hydroxychloroquine (HCQ) in a placebo-controlled fashion to prevent clinical RA. A subset of 36 individuals (24 Converters and 12 Non-Converters) was profiled using single-cell ASAP-seq and 137-plex CITE-seq, including paired single-cell TCR and BCR sequencing. Another subset of 100 individuals (47 Converters and 53 Non-Converters) was analyzed with mass cytometry (MDIPA panel) across 1–3 visits. B, Time points of sample collection per individual. The time to clinical RA diagnosis (day 0) is shown for Converters, and a follow-up visit is indicated for Nonconverters. Data are presented separately for individuals analyzed by CITE-seq/scTCR-seq/scBCR-seq/scASAP-seq (left) and those analyzed by mass cytometry (right). Colored points represent visit status: baseline/follow-up (green) or post-RA (purple). C, Broad immune cell-type identification presented in the UMAP, showing major immune cell types identified across CITE-seq, scASAP-seq, and mass cytometry datasets. D, Strategy to integrate CITE-seq and mass cytometry data for T cell analysis. UMAP projection of integrative T cell subclusters projected into a shared embedding space using overlapping measured 33 proteins with CITE-seq data as reference and mass cytometry as query. Clusters in CITE-seq and joint-space are color-coded and labeled consistently across modalities. E, Normalized protein expression in joint T cell subclusters from CITE-seq (left) and mass cytometry data (right). F, Schematic of the predictive modeling approach. Two research questions were addressed: (RQ1) distinguishing Converters from Nonconverters at baseline (V0) and (RQ2) predicting time to RA onset among Converters. Predictors such as Tph proportions were incorporated into random forest model to prioritize them. Note: CyTOF analyses included study participants who received HCQ or placebo, while CITE-seq and ASAP-seq only included participants who received placebo.
.jpg?1) Fig. 2: T cell subcluster analysis by integrating single-cell datasets from CITE-seq, mass cytometry, scASAP-seq, and TCR. A, B, Generalized linear mixed model (GLMM) analysis comparing RA Converters and Nonconverters at each visit point. The odds ratio and 95% confidence intervals (CIs) for each T cell subcluster are shown separately for CD4+ T cells (left) and CD8+ T cells (right). Differences in datasets (CITE-seq or CyTOF), age, gender, batch, individual, and HCQ were corrected by including them as covariates. A, Comparison at baseline (V0). B, Comparison after RA onset (V1). C, D, Differentially expressed genes in CD4+ and CD8+ T cells between Converters and Nonconverters at baseline (V0) and post-RA (V1) stages. T-cell marker genes are annotated. E, Graphical representation of the Converter-related peak analysis. Open chromatin regions associated with RA conversion were identified by comparing chromatin accessibility between Converters and Nonconverters at baseline (V0) and post-RA (V1) stages. F, Venn diagram illustrating the number of significant peaks identified at V0 and V1 (adjusted p-value < 0.05). G, UMAP plot showing the expression of PTPN22, a Converter-associated locus, identified at V0 using CITE-seq data. H, Violin plot showing the PTPN22 mostly expressed by NK cells. I, Illustration of a Converter-associated open chromatin region within the PTPN22 locus. J, UMAP projection of T cells in CITE-seq data colored by CD4T (purple) and CD8T cells (green). K, Clonal expansion of TCR repertoires across visit points. L, M, The composition of the top 20 expanded T cell clones is shown for CD4+ T cells and CD8+ T cells at V0 and V1. The dominant Tph (T-14) clones are highlighted in Converters and Nonconverters.
Fig. 2: T cell subcluster analysis by integrating single-cell datasets from CITE-seq, mass cytometry, scASAP-seq, and TCR. A, B, Generalized linear mixed model (GLMM) analysis comparing RA Converters and Nonconverters at each visit point. The odds ratio and 95% confidence intervals (CIs) for each T cell subcluster are shown separately for CD4+ T cells (left) and CD8+ T cells (right). Differences in datasets (CITE-seq or CyTOF), age, gender, batch, individual, and HCQ were corrected by including them as covariates. A, Comparison at baseline (V0). B, Comparison after RA onset (V1). C, D, Differentially expressed genes in CD4+ and CD8+ T cells between Converters and Nonconverters at baseline (V0) and post-RA (V1) stages. T-cell marker genes are annotated. E, Graphical representation of the Converter-related peak analysis. Open chromatin regions associated with RA conversion were identified by comparing chromatin accessibility between Converters and Nonconverters at baseline (V0) and post-RA (V1) stages. F, Venn diagram illustrating the number of significant peaks identified at V0 and V1 (adjusted p-value < 0.05). G, UMAP plot showing the expression of PTPN22, a Converter-associated locus, identified at V0 using CITE-seq data. H, Violin plot showing the PTPN22 mostly expressed by NK cells. I, Illustration of a Converter-associated open chromatin region within the PTPN22 locus. J, UMAP projection of T cells in CITE-seq data colored by CD4T (purple) and CD8T cells (green). K, Clonal expansion of TCR repertoires across visit points. L, M, The composition of the top 20 expanded T cell clones is shown for CD4+ T cells and CD8+ T cells at V0 and V1. The dominant Tph (T-14) clones are highlighted in Converters and Nonconverters.
.jpg?1) Fig. 3: Predictive modeling for RA conversion. A, ROC analysis of a composite predictive model incorporating multiple predictors using samples available CyTOF or CITE-seq data (47 Converters and 53 Nonconverters). For cell type frequencies such as Tph cells, values from CITE-seq were prioritized. For samples lacking CITE-seq data, cell type frequencies predicted via integration— as described in Figures 1—were used instead. B, Decision tree model for RA conversion. A classification tree showing hierarchical decision rules based on CCP3 levels, Tph cells (T-14), and RF status to predict RA conversion risk. Pie charts at terminal nodes indicate the proportion of Converters (blue) and Nonconverters (gray). C-D, Survival analysis of RA conversion stratified by Tph and CCP3 levels. Kaplan-Meier curves showing time to RA onset based on (C) top and bottom 20% of Tph proportions and (D) top and bottom 20% of CCP3 levels. Statistical significance was assessed using log-rank tests.
Fig. 3: Predictive modeling for RA conversion. A, ROC analysis of a composite predictive model incorporating multiple predictors using samples available CyTOF or CITE-seq data (47 Converters and 53 Nonconverters). For cell type frequencies such as Tph cells, values from CITE-seq were prioritized. For samples lacking CITE-seq data, cell type frequencies predicted via integration— as described in Figures 1—were used instead. B, Decision tree model for RA conversion. A classification tree showing hierarchical decision rules based on CCP3 levels, Tph cells (T-14), and RF status to predict RA conversion risk. Pie charts at terminal nodes indicate the proportion of Converters (blue) and Nonconverters (gray). C-D, Survival analysis of RA conversion stratified by Tph and CCP3 levels. Kaplan-Meier curves showing time to RA onset based on (C) top and bottom 20% of Tph proportions and (D) top and bottom 20% of CCP3 levels. Statistical significance was assessed using log-rank tests.
Disclosures: J. Inamo: None; A. Bylinska: None; M. Smith: None; L. Vanderlinden: None; C. Wright: None; T. Stephens: None; M. Feser: None; C. Striebich: None; J. O'Dell: None; J. Sparks: Boehringer Ingelheim, 5, Bristol-Myers Squibb (BMS), 5, Janssen, 5; J. Davis: Pfizer, 5, Remission Medical, 1, 9, 10, Rheumasense, 1, 9, 10; J. Graf: None; M. McMahon: Artiva, 2, AstraZeneca, 2, 6, Aurinia, 6, Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 1, 5, 6, Novartis, 5, Roche, 1; E. Solow: None; L. Forbess: Novartis, 5; A. Tiliakos: None; D. Fox: None; M. I. Danila: None; D. Lewis. Horowitz: None; J. Kay: Biogen, 5, Celltrion, Inc., 2, Gate Bioscience, 2, Immunitas Therapeutics, 2, Immunovant, Inc., 2, Istesso, Ltd, 2, Kolon TissueGeje, Inc, 1, Organon, LLC, 2, Sana Biotechnology, Inc., 2, Santa Ana Bio, Inc., 2, Spyre Therapeutics, 2, Wolters Kluwer NV, 9; J. James: GlaxoSmithKlein(GSK), 2, Progentec, 5; V. Holers: Q32 Bio, 12,, 2; K. Deane: Inova Diagnostics, 1; J. Guthridge: None; F. Zhang: None.
To cite this abstract in AMA style:
Inamo J, Bylinska A, Smith M, Vanderlinden L, Wright C, Stephens T, Feser M, Striebich C, O'Dell J, Sparks J, Davis J, Graf J, McMahon M, Solow E, Forbess L, Tiliakos A, Fox D, I. Danila M, Lewis. Horowitz D, Kay J, James J, Holers V, Deane K, Guthridge J, Zhang F. Longitudinal peripheral blood multi-omic profiling in at-risk individuals uncovers immune signatures and predictive models for future rheumatoid arthritis conversion [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-peripheral-blood-multi-omic-profiling-in-at-risk-individuals-uncovers-immune-signatures-and-predictive-models-for-future-rheumatoid-arthritis-conversion/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-peripheral-blood-multi-omic-profiling-in-at-risk-individuals-uncovers-immune-signatures-and-predictive-models-for-future-rheumatoid-arthritis-conversion/
