Session Information
Date: Sunday, November 12, 2023
Title: (0325–0344) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoids (GCs) continue to be the cornerstone of therapy for many rheumatic diseases, though long-term exposure to GCs has been linked to dozens of potential toxicities. The Glucocorticoid Toxicity Index (GTI) is a validated instrument that quantifies change in GC toxicity longitudinally. The GTI produces two scores: the Cumulative Worsening Score (CWS) and the Aggregate Improvement Score (AIS), which are calculated by evaluation of 9 domains involving clinical, laboratory, and imaging information. We developed a prospective cohort of patients with rheumatic diseases receiving long-term GCs with the goals of assessing longitudinal GC toxicity as measured by the GTI, quality of life (QoL), and healthcare resource utilization to better quantify the personal and societal costs of glucocorticoids.
Methods: We enrolled adults ages 18-89 with any rheumatic disease diagnosis, receiving a GC taper with a starting dose of ≥7.5 mg/day (prednisone or equivalent dose) over an anticipated period of ≥ 3 months. Visits are conducted at baseline, 6, and 12 months and involve assessment of GTI scores, GC use and exposure, patient-reported outcomes including the EQ-5D Visual Analogue Scale [VAS], assessment and quantification of healthcare resource utilization both within Mass General Brigham (MGB) (assessed by an investigator or study coordinator by review of electronic health records) and outside of MGB (assessed by patient survey). Data collection was performed in REDCap and the Steritas Cloud GTI app. Patients with available 6-month data are included here. Differences between EQ-5D VAS scores and physician clinic visits were assessed for those with GTI scores above vs. below the median using linear regression, with and without adjustment for disease activity as assessed by Routine Assessment of Patient Index Data 3 (RAPID3) scores. Potential GTI CWS scores ranged from 0 (indicating no toxicity) to 439 (indicating the maximum measurable GC toxicity at 6 months).
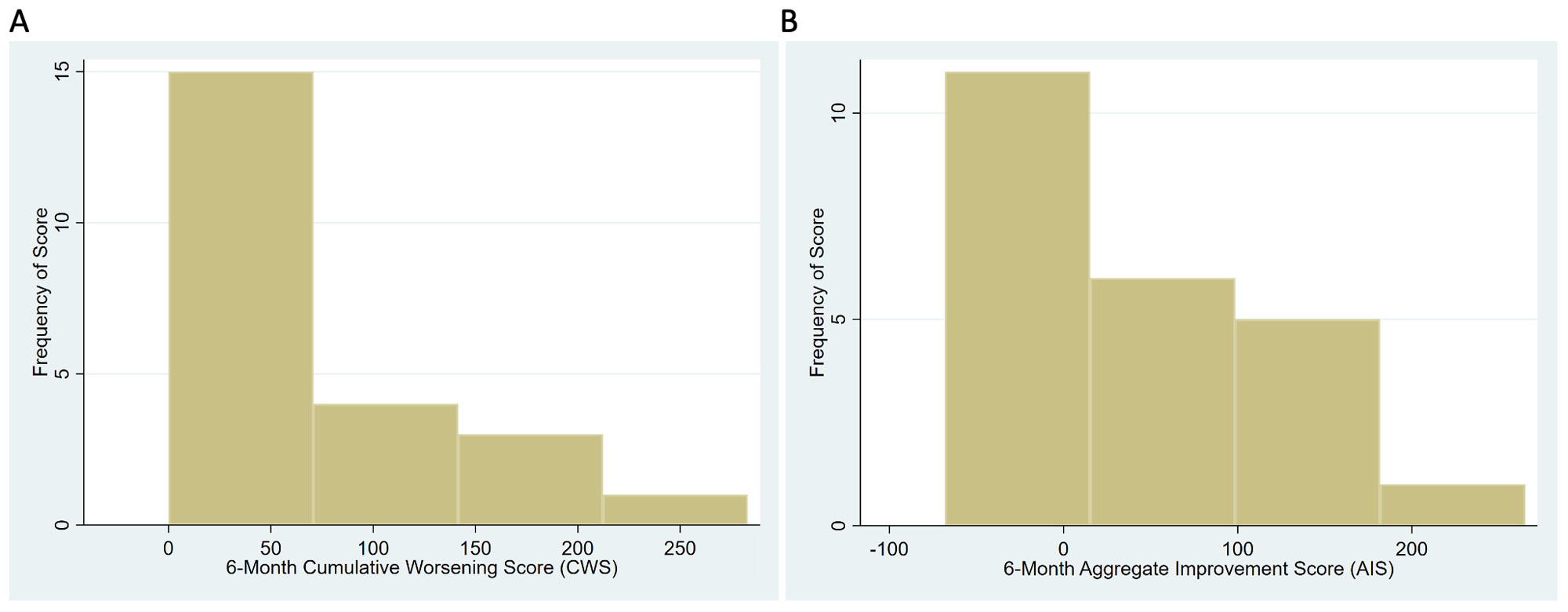
Results: Twenty three patients with available 6-month data were included. The 6-month median (IQR) GTI CWS was 63 (29, 121) (Figure). The 6-month median (IQR) GTI AIS was 21 (-19, 113). 21 out of 23 patients (91.3%) had CWS values > 0 at 6 months. The most common individual GTI domains in which patients had an increase in toxicity included Neuropsychiatric (14/23; 61%), Skin (11/23; 48%), Blood Pressure (9/23; 39%), and Body Mass Index (4/23; 17%). The Infection, Myopathy, and Lipid Metabolism domains each indicated toxicity in 3/23 (13%) of patients, and the Glucose Tolerance domain in 2/23 (9%). After adjusting for RAPID3 scores, those with 6-month CWS values > median had 2.7 more clinic visits (p=0.26) and EQ-5D VAS scores that were 2.1 points higher (p=0.72) than those with CWS values < median.
Conclusion: Almost all patients had some degree of GC toxicity at 6 months, based on their GTI scores. Patients with higher GC toxicity had numerically more clinic visits. Quantifying GC toxicity as well as its impact on factors important to both patients and society will be critical to assessing the benefits and cost-effectiveness of steroid-sparing medications moving forward.
To cite this abstract in AMA style:
Patel N, McMahon A, McMahon G, Perez-Espina S, Jha I, Jarvie A, Stone J. Longitudinal Glucocorticoid Toxicity in Rheumatic Disease Patients (LONG-TOX) and Associations with Quality of Life and Healthcare Resource Utilization: Interim Analysis from a Prospective Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/longitudinal-glucocorticoid-toxicity-in-rheumatic-disease-patients-long-tox-and-associations-with-quality-of-life-and-healthcare-resource-utilization-interim-analysis-from-a-prospective-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-glucocorticoid-toxicity-in-rheumatic-disease-patients-long-tox-and-associations-with-quality-of-life-and-healthcare-resource-utilization-interim-analysis-from-a-prospective-cohort/