Session Information
Date: Sunday, November 12, 2023
Title: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Cell-bound complement activation products (CB-CAPs), when part of a multi-analyte assay with algorithm (MAP), are valuable SLE diagnostic biomarkers. Levels of erythrocyte-bound complement activation products have been associated with SLE disease activity. The clinical and serologic phenotype of longitudinal MAP-positive patients has not been well described. Herein, we assessed the relationship between longitudinal MAP results with clinical and laboratory variables.
Methods: This was a longitudinal study of adult SLE patients (2012 SLICC or ACR/EULAR criteria) with ≥2 routine clinic visits from June 2020 to July 2022. Patients completed the polysymptomatic distress scale. The rheumatologist scored the PGA and SLEDAI scores. Autoantibodies including ANA and anti-RNA binding proteins were measured by ELISA. Anti-dsDNA was measured by immunofluorescence using the Crithidia luciliea assay. CB-CAPs were analyzed by flow cytometry. The multi-analyte assay panel (MAP) was determined using a 2-tier algorithm. Chi-square and ANOVA tests were used to analyze differences in demographic and disease history between persistently MAP positive, MAP negative, and patients with changing MAP positivity. Serologies and clinical variables at follow-up visits were compared using generalized linear models.
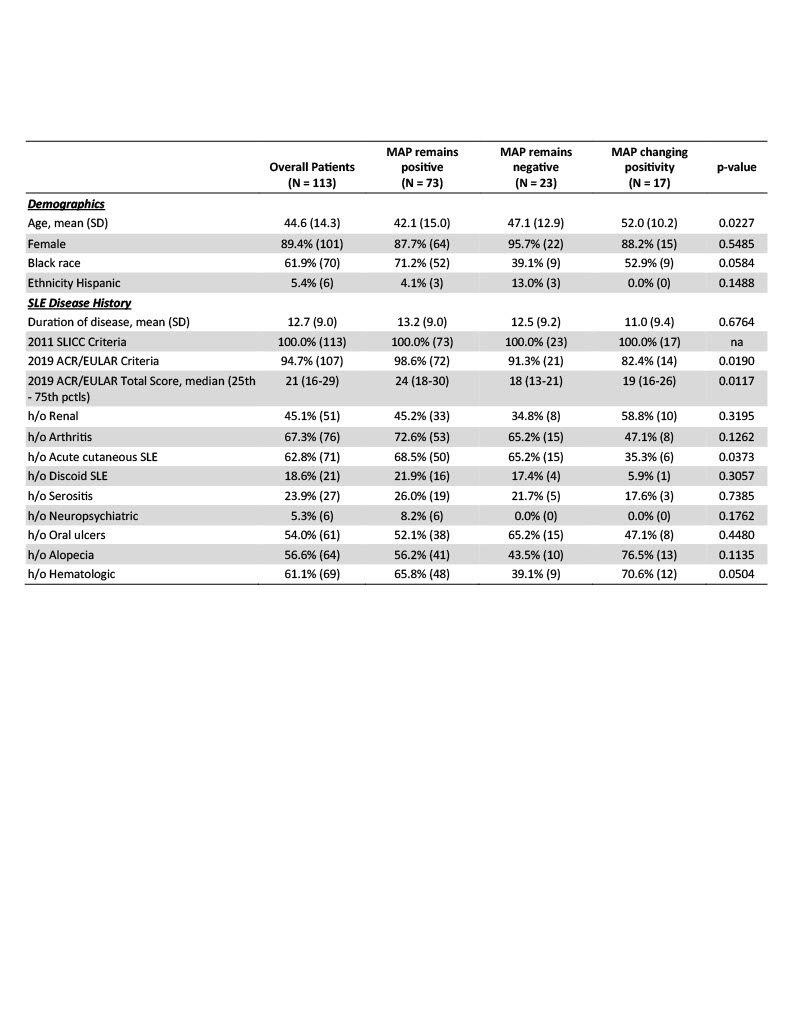
Results: In this longitudinal cohort of 113 patients with 175 follow-up visits (90% female, 62% Black, mean age 45) 65% were consistently MAP positive, 20% were negative, and MAP positivity changed in 15%. Persistent MAP-positive patients were younger and more often of the Black race. There was no difference in MAP positivity based on disease duration. Significantly more MAP-positive patients met ACR/EULAR criteria and had higher total ACR/EULAR scores. Patients who remained MAP positive were more likely to have a history of acute cutaneous lupus but there was no difference in other historical manifestations between groups (Table 1).
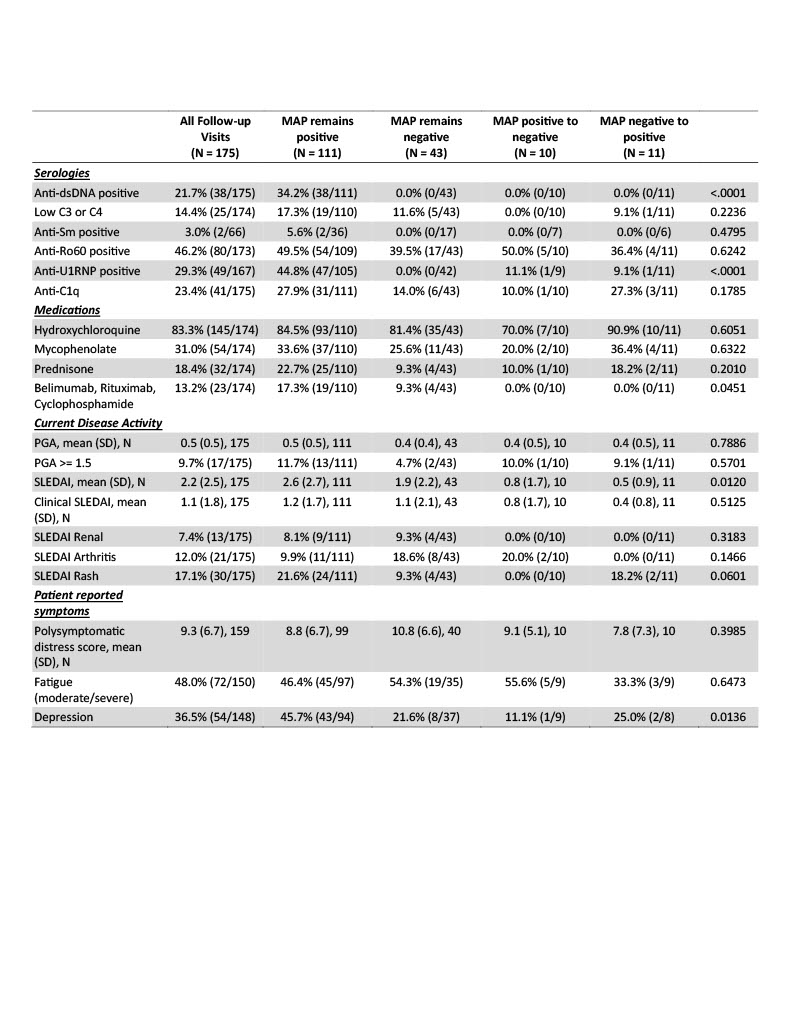
When evaluating longitudinal associations, patients with persistent MAP positivity had higher total SLEDAI scores, but there was no difference in the clinical SLEDAI. Therapy was comparable across groups except for greater use of belimumab, rituximab, and cyclophosphamide in persistent MAP-positive patients. Patients who remained MAP positive reported higher rates of depression, but a similar burden of polysymptomatic distress and fatigue. A greater number of lupus-specific serologies were present in those with MAP positivity (Table 2).
Conclusion: Identifying endotypes of SLE is key to advancing personalized medicine. In this cohort of patients with SLE, most patients had static MAP results. MAP results changed between visits in a subset of patients; although there was not a distinct clinical, demographic, or laboratory phenotype in those patients. Patients with consistent MAP positivity reported more depression and had a greater burden of disease activity as measured by the ACR/EULAR score and greater use of biologic and cytotoxic therapy. Combining longitudinal MAP scores with assessments of SLE activity may provide important prognostic information. Larger studies are ongoing to evaluate the relationship between individual CB-CAPs and disease activity.
To cite this abstract in AMA style:
Rogers J, Eudy A, Wojdyla D, O'Malley T, Pisetsky D, Alexander R, Sun K, Criscione-Schreiber L, Doss J, Sadun R, Maheswaranathan M, Clowse M. Longitudinal Evaluation of Cell-bound Complement Activation Products in Patients with SLE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/longitudinal-evaluation-of-cell-bound-complement-activation-products-in-patients-with-sle/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-evaluation-of-cell-bound-complement-activation-products-in-patients-with-sle/