Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: For patients with Rheumatoid Arthritis (RA) who do not achieve adequate clinical response with combined conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), the next step in goal-directed therapy is initiation of either biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs). bDMARDs include tumour-necrosis factor inhibitors (TNFi) or non-TNFi classes. Since inception of Ontario Best Practice Research Initiative (OBRI), new treatment options have become available. We aimed to describe the evolution of relative use of non-TNFi vs. TNFi in Ontario-based practices from 2008-2017.
Methods: Adult patients with RA enrolled in the OBRI who started therapy with bDMARDs or tsDMARDs anytime during, or up to 30 days before, enrollment were included. Using descriptive analysis of data from each year between 2008 and 2017, the relative proportion of the population treated with TNFi and non-TNFi therapy was measured for (i) all patients and (ii) those initiating their first bDMARD/tsDMARD. TNFi included: Etanercept, Adalimumab, Certolizumab, Golimumab, and Infliximab. Non-TNFi included: Abatacept, Rituximab, Tocilizumab, and Tofacitinib.
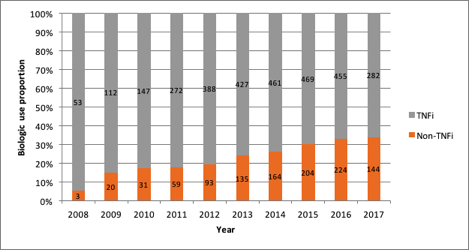
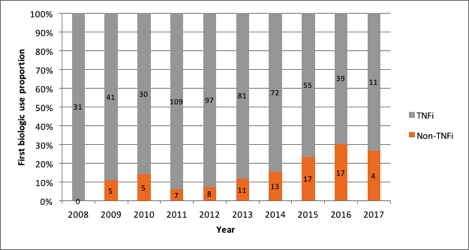
Results: A total of 1,057 patients were included of whom 653 were bDMARD/tsDMARD na•ve. In 2008, the relative non-TNFi use was 3/56 (5.4%) in all patients and 0/31 (0%) in treatment-na•ve patients. By 2013 the proportion non-TNFi use increased to 135/562 (24%) in all patients and 11/92 (12.0%) in treatment-na•ve patients. This increasing trend in relative non-TNFi utilization continued in both groups until 2016 when relative use was 224/679 (33.0%) in all patients and 17/56 (30.4%) in treatment-na•ve. This was followed by 144/426 (33.8%) and 4/15 (26.7%), respectively in 2017.
Conclusion: This descriptive analysis of data from the OBRI cohort shows an increase in the use of non-TNFi therapies. The overall trend towards greater use of non-TNFi therapies as first line agents after combined csDMARDs may be partially explained by the presence of guidelines that allow clinicians to select any of the above options as first line advanced therapies. Future analyses evaluating patient-, disease- and concomitant drug use-specific determinants of physician decision-making will be conducted.
Figure 1: Proportion of biologic use according to mechanism of action by calendar year (n=1057)
Figure 2: Proportion of first biologic use in biologic na•ve patients according to mechanism of action by time period (n=653)
To cite this abstract in AMA style:
Hepworth E, Movahedi M, Rampakakis E, Mirza R, Lau A, Cesta A, Pope JE, Bombardier C. Longitudinal Changes in Relative Market Share Proportions of Biologic and Targeted Synthetic Disease-Modifying Anti-Rheumatic Drugs for Treatment of Rheumatoid Arthritis: Descriptive Data from the Ontario Best-Practice Research Initiative Database [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/longitudinal-changes-in-relative-market-share-proportions-of-biologic-and-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-for-treatment-of-rheumatoid-arthritis-descriptive-data-from-the-ont/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-changes-in-relative-market-share-proportions-of-biologic-and-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-for-treatment-of-rheumatoid-arthritis-descriptive-data-from-the-ont/