Session Information
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Although anti-beta2 glycoprotein is one of the three antiphospholipid antibodies recognized in the Sydney APS classification criteria, it is one of the least studied. Lupus anticoagulant, the antiphospholipid antibody most associated with both thrombosis and adverse pregnancy outcomes, can be divided into two subsets: those that target beta2 glycoprotein I and those that target prothrombin (Blood 1995;86:617-23). In SLE, lupus anticoagulant can fluctuate over time, making it difficult to define which patient is “persistently positive”. Fluctuation could reflect change in SLE activity, change in SLE treatement, or both. We looked at two definitions of “positive”: 20 (medium) as in the classification criteria; or 40 as part of “triple positivity”. We sought to address some of these questions in our prospective SLE cohort.
Methods: The SLE patients met revised ACR and/or SLICC classification criteria. Anti-beta 2 glycoprotein IgG, IgM, and IgA were measured by ELISA (INOVA) at 2,393 visits in 1,417 patients. The patients were 92% female, 50% Caucasian, and 40% African-American. Forty-three percent of the patients had more than 1 assessment (57% had 1, 26% had 2, 10% had 3, and 7% had 4 or more assessments).
Results: Anti-beta 2 glycoprotein IgG > 20 (medium positive) or > 40 (high positive) was more common in Caucasians than in African-Americans (p=0.0012, 0.0089) but IgA > 20 or > 40 was more common in African-Americans (p=NS). Table 1 shows that about 40-76% of the time a patient who was positive at the first check became negative at the second check (but the converse was not true: most patients initially negative remained negative). Table 2 shows the effect of testing over multiple visits. IgA remained the most frequent isotype. There was minimal incremental value in serial testing overall. Table 3 shows a multiple variable model for the isotype (IgG) most associated with thrombosis. IgG anti-beta 2 glycoprotein was clearly less frequent in African-Americans and was also clearly reduced in those taking hydroxychloroquine. There was borderline evidence, in contrast, that it might be reduced by immunosuppression treatment (p=0.06). A similar multivariate model of IgA anti-beta 2 glycoprotein showed that hydroxychloroquine also reduced IgA (OR 0.72, 95% CI 0.58, 0.90; p=0.004).
Conclusion: Anti-beta 2 glycoprotein IgG is more common in Caucasian than African-American SLE, but the opposite is true for the IgA isotype. As with other antiphospholipid antibodies in SLE, there is considerable fluctuation over visits, with 40-76% becoming negative at a second check. Hydroxychloroquine use reduced both the IgG and IgA isotypes, again reinforcing the benefit of hydroxychloroquine in thrombosis reduction. The IgA anti-beta 2 glycoprotein finding is particularly important, in that hydroxychloroquine did not reduce IgA anticardiolipin (Lupus Sci Med 2016;3:e000107).
 Proportion who changed status from first to second visit.
Proportion who changed status from first to second visit.
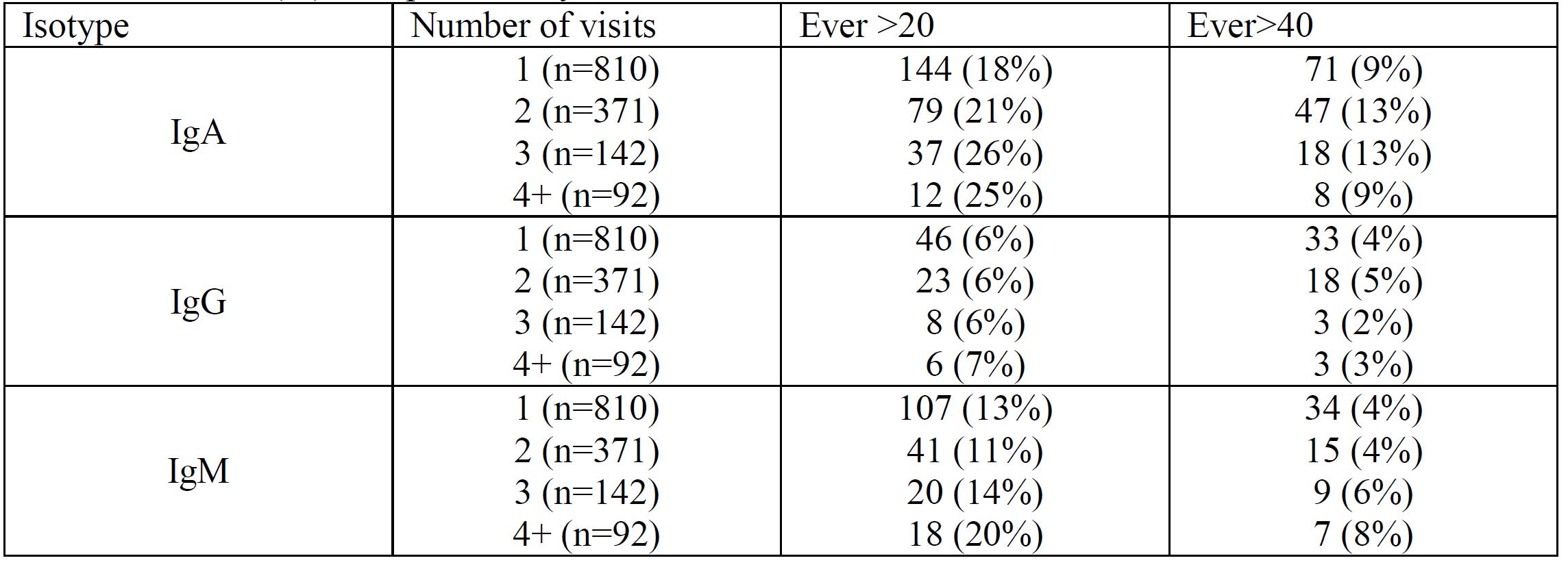 Number (%) ever positive, by number of assessments.
Number (%) ever positive, by number of assessments.
 Multivariate model for association between predictors and Anti-beta 2 IgG
Multivariate model for association between predictors and Anti-beta 2 IgG
To cite this abstract in AMA style:
Petri M, Magder L, Goldman D. Longitudinal Assessment of Anti-beta 2 Glycoprotein in SLE [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/longitudinal-assessment-of-anti-beta-2-glycoprotein-in-sle/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-assessment-of-anti-beta-2-glycoprotein-in-sle/
