Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Primary Sjögren’s syndrome (pSS) is a systemic autoimmune rheumatic disease characterised by sicca features and systemic manifestations such as pain and fatigue. The classic therapeutic approach for pSS is based upon symptomatic treatment of sicca manifestations with immunosuppression for more severe disease. To date, there has been no longitudinal analysis of medication use for patients with pSS.
Methods: We used data from the United Kingdom Primary Sjögren’s Syndrome Registry to identify patients with sufficient follow-up data including their current medications. Patients who were not on medication at visit 1 but who were on medication at visit 2 were included. Those patients who were not on medication at visit 1 or visit 2 were included for comparison. The medication regimes were group into four categories: Hydroxycholoroquine (HCQ), Immunosuppression, NSAIDs and Analgesics. The differences in characteristics and patient-reported scores between each group were evaluated by analysis of variance. Paired data between the two visits was evaluated by a paired t-test.
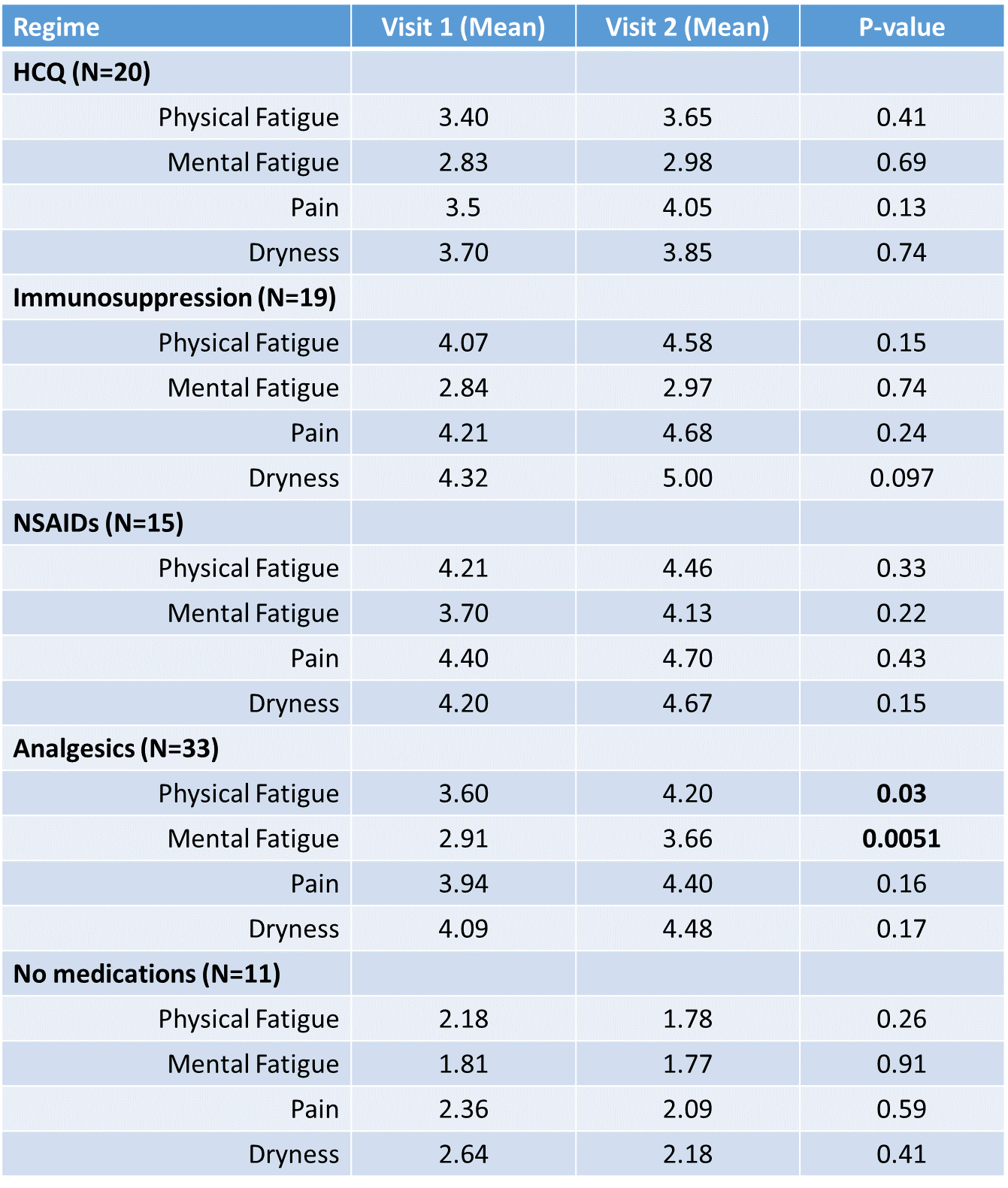
Results: 98 patients had sufficient data to be included. The median time between visits was 4 years and was not different between groups (p=0.48). Patient-reported scores at visit 1 revealed significant differences in physical fatigue (p=0.017) and pain (p=0.049) between the groups. Patients treated with HCQ, Immunosuppression, NSAIDs or Analgesia (N=87) showed a worsening of physical and mental fatigue, pain and dryness whereas the inverse was true for those patients not treated (N=11). In particular, patients who were started on analgesics reported higher physical fatigue (p=0.03), mental fatigue (p=0.005) and pain (p=0.16) at visit 2. Haematological analysis reveal that patients treated with HCQ had a lower ESR (p=0.045), IgG (p=0.0044) and IgM (p=0.0031) whereas those treated with other immunosuppresives only had a lower IgM (p=0.0035).
Conclusion: Whilst HCQ appears to reduce the clinical parameters associated with active disease in patients with pSS, it was not accompanied by improvement in patient-reported symptoms at visit 2. Symptomatic treatments in the form of NSAIDs or analgesics appear to be associated with worsening patient-reported scores whereas those not treated with any medication seem to improve at visit 2. Despite the limitations with this analysis our data suggest that HCQ, immunosuppressive therapies and symptomatic medications are ineffective in improving patients’ symptoms.
Figure 1: Patient Reported Scores at Visit 1 and Visit 2 with p-values determined using the paired t-test.
To cite this abstract in AMA style:
Davies K, Mirza K, Tarn J, Regan M, Vadivelu S, Clunie G, Andrews J, Price E, Young-Min S, Giles I, Dasgupta B, Lawson C, Gendi N, McHugh NJ, Bombardieri M, Pitzalis C, Sutcliffe N, Bowman S, Lendrem D, Ng WF. Longitudinal Analysis of Different Therapeutic Strategies in Patients with Primary Sjögren’s Syndrome [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/longitudinal-analysis-of-different-therapeutic-strategies-in-patients-with-primary-sjogrens-syndrome/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-analysis-of-different-therapeutic-strategies-in-patients-with-primary-sjogrens-syndrome/