Session Information
Date: Sunday, November 8, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes II: Bench to Bedside (1507–1511)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Anti-nuclear antibodies (ANA) are important biomarkers for the diagnosis and classification of systemic lupus erythematosus (SLE). However, emerging data from cross-sectional studies suggest variation in the performance between ANA assays. The purpose of this project was to compare the performance of three different ANA assays in a longitudinal analysis of samples from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort.
Methods: We used demographic, clinical, and serological data of SLICC patients who fulfilled the 1997 Updated ACR SLE Classification Criteria and were enrolled within 15 months of diagnosis. Samples from enrolment and follow-up visits at years 3 and 5, were assayed using three FDA-approved ANA tests, including two HEp-2 indirect immunofluorescence assays, IFA1 (BioRad, Hercules, USA) and IFA2 (NovaLite, Inova Diagnostics, San Diego, USA), and an enzyme-linked immunosorbent assay (ELISA) (Inova Diagnostics, San Diego, USA), at one central laboratory (Calgary, AB). A positive test was defined as a titer of ≥1:80 for IFA1 and IFA2 and ≥20 chemiluminescent units (CU) for the ELISA. The frequency of positivity, titer and patterns among different ANA assays were compared using weighted kappa statistics (к) and McNemar tests. Longitudinal analysis of ANA titers was assessed for IFA1 and IFA2 using a mixed-effects restricted maximum likelihood regression model fitting a quadratic trend with unstructured co-variance. ANA patterns were categorized into three groups: 1) pure nuclear (e.g. homogeneous, speckled), 2) cytoplasmic and mitotic patterns (e.g. dense fine speckled and centrosome), and 3) mixed nuclear, cytoplasmic and mitotic patterns.
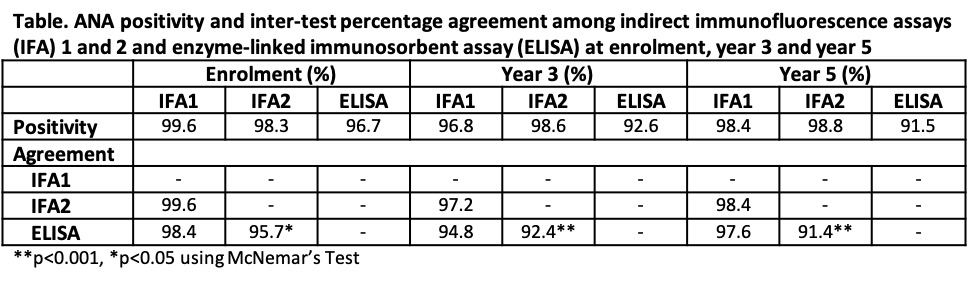
Results: 806 patients were included; 88.7% were female and mean age at diagnosis was 35.2 (SD 13.6) years. At enrolment, the frequency of ANA positivity by IFA1, IFA2, and ELISA was high (99.6%, 98.3%, and 96.7% respectively) (Table) and there was strong agreement between IFA1 and IFA2 (99.6% agreement) and IFA1 and ELISA (98.4%), but significant differences were detected for IFA2 and ELISA (95.7% agreement, p< 0.05 on McNemar’s test). Over five years of follow-up, the prevalence and agreement in ANA positivity remained high for IFA1 and IFA2, decreasing slightly only for ELISA (91.5% positivity and 91.4% agreement with IFA2 at year 5). There was fair to moderate agreement between IFA1 and IFA2 titres at enrolment (84.7% agreement, к=0.52), year 3 (77.0%, к=0.32), and year 5 (80.1%, к=0.37). A quadratic trend model revealed that IFA1 titers decreased more rapidly compared to IFA2 (p< 0.001) (Figure 1). There was fair to moderate agreement between IFA1 and IFA2 ANA patterns at enrolment (75.4% agreement, к=0.45), year 3 (66.7%, к=0.28), and year 5 (69.4%, к=0.37) (Figure 2). Pure nuclear patterns were the most common, followed by mixed patterns.
Conclusion: In recent-onset SLE, early ANA positivity is 96.7-99.6% depending on the test. Agreement between the two IFA’s was high at diagnosis and sustained over five years but was lower between one of the IFA’s and ELISA. While titers did decrease slightly over time, ANA patterns remained largely unchanged. A future study with a longer follow-up period and clinical correlation is underway.
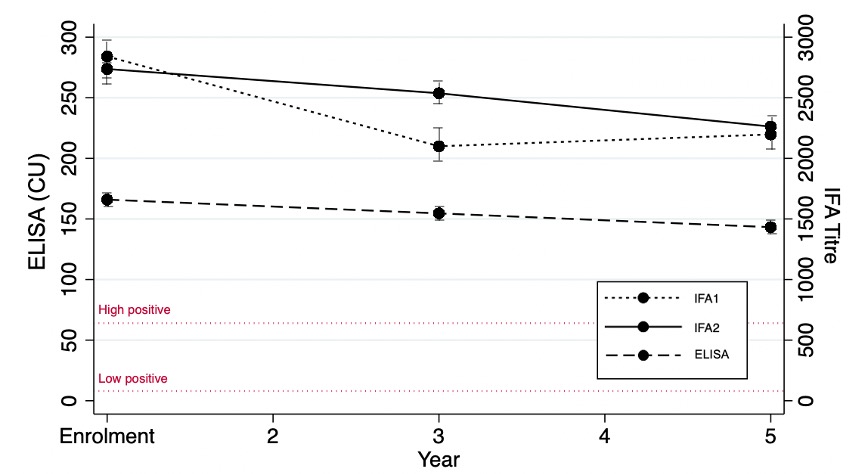 Figure 1. ANA titres decrease over time with indirect immunofluorescence assay (IFA) 1, IFA2, and enzyme-linked immunosorbent assay (ELISA) in chemiluminescent units (CU).
Figure 1. ANA titres decrease over time with indirect immunofluorescence assay (IFA) 1, IFA2, and enzyme-linked immunosorbent assay (ELISA) in chemiluminescent units (CU).
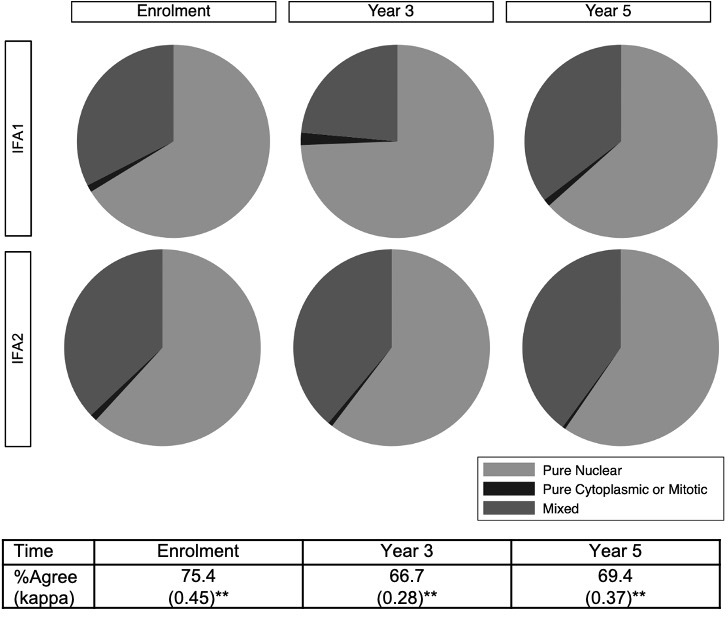 Figure 2. ANA patterns over time with indirect immunofluorescence assays (IFA) 1 and 2. **p < 0.0001 using weighted kappa statistics.
Figure 2. ANA patterns over time with indirect immunofluorescence assays (IFA) 1 and 2. **p < 0.0001 using weighted kappa statistics.
To cite this abstract in AMA style:
Choi M, Clarke A, Costenbader K, Urowitz M, Hanly J, Gordon C, St. Pierre Y, Bae S, Romero-Díaz J, Sanchez-Guerrero F, Bernatsky S, Wallace D, Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Bruce I, Petri M, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jönsen A, Alarcón G, Van Vollenhoven R, Aranow C, Mackay M, Ruiz-Irastorza G, Lim S, Inanc M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Fritzler M. Longitudinal Analysis of ANA Assay Performance in SLE from the SLICC Inception Cohort [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/longitudinal-analysis-of-ana-assay-performance-in-sle-from-the-slicc-inception-cohort/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-analysis-of-ana-assay-performance-in-sle-from-the-slicc-inception-cohort/