Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tocilizumab (TCZ)—an IL-6 receptor inhibitor—has demonstrated efficacy in improving signs/symptoms, reducing joint damage, and improving physical function in rheumatoid arthritis (RA) patients (pts). This analysis assessed the long-term safety of TCZ (up to 5.8 y of exposure) in adult RA pts.
Methods: Analysis was performed in all pts who received ≥1 TCZ dose in 5 placebo-controlled trials (OPTION, TOWARD, RADIATE, AMBITION, LITHE), a clinical pharmacology study, or long-term extension studies. Data were pooled and analyzed from initial TCZ exposure to April 1, 2011 (cutoff).
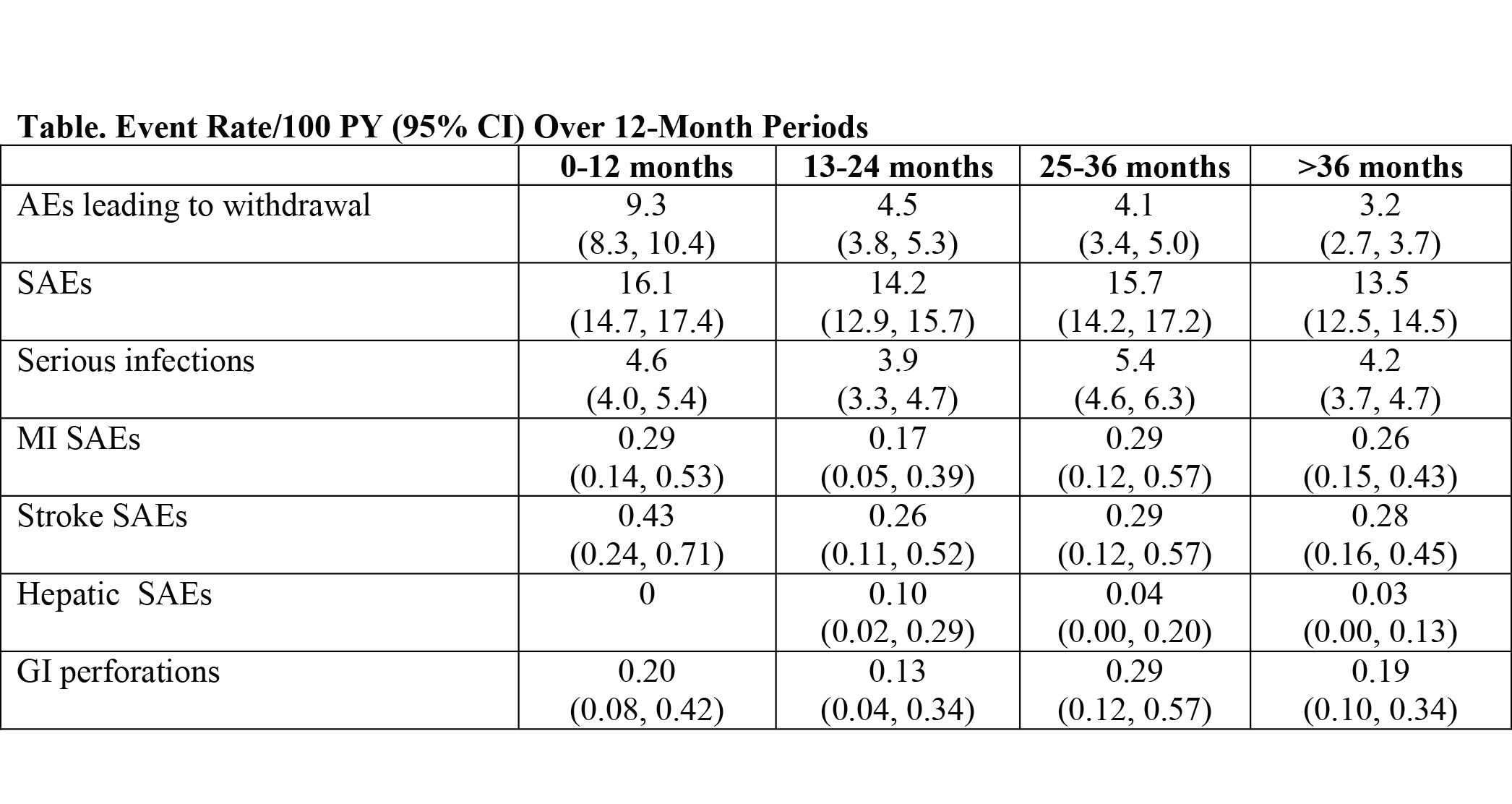
Results: 4009 pts were included. Mean (median [range]) duration was 3.7 (4.6 [0.0-5.8]) y; total observation time was 14,994 pt-y (PY). Rates of serious adverse events (SAEs), serious infections, myocardial infarction (MI) SAEs, stroke SAEs, hepatic SAEs, and gastrointestinal (GI) perforations were stable over time (Table). The overall rate of AEs leading to withdrawal was 5.0/100PY (95% CI: 4.7, 5.4). Infections, laboratory abnormalities, and neoplasms were the most common AEs leading to withdrawal (0.97/100PY, 0.89/100PY, and 0.80/100PY). 8 pts withdrew because of anaphylaxis events; these were previously reported.1 Rates/100PY (95% CI) were 14.6 (14.0, 15.3) for SAEs and 0.57 (0.45, 0.70) for deaths. The most common SAEs were infections, which occurred at a rate of 4.5/100 PY (95% CI: 4.1, 4.8); the most common serious infection was pneumonia (0.95/100 PY; 95% CI: 0.80, 1.12). Overall rates/100PY (95% CI) of MI SAEs, stroke SAEs, and hepatic SAEs were 0.25 (0.18, 0.35), 0.31 (0.23, 0.42), and 0.04 (0.01, 0.09), respectively. The GI perforation rate was 0.20/100PY (95% CI: 0.13, 0.29). There were 194 confirmed malignancies, including 65 nonmelanoma skin cancer (NMSC) cases, corresponding to an overall rate/100PY (95% CI) of 1.29 (1.12, 1.49) and, excluding NMSC, of 0.86 (0.72, 1.02). SIR for malignancies (all sites) was 1.19 (0.99, 1.42), which was not statistically different from the US general population rate (SEER database).
Conclusion: The safety profile of TCZ in the current analysis is consistent with that in prior TCZ analyses1,2; it remained stable over a mean treatment duration of 3.7 y, and no new safety signals have emerged. AE rates described herein are consistent with those reported in the RA population, and the overall rate of malignancies does not exceed reported background rates (SEER database).3-10
References
1. Schiff Arthritis Res Ther 2011;13:R141; 2. Genovese. ACR 2011. Abstract 2217; 3. Curtis Arthritis Rheum 2011;63:346; 4. Curtis Ann Rheum Dis 2011;70:1401; 5. Galloway Rheumatology 2010;50:124; 6. Dixon Rheumatology 2006;45(supp1):i2; 7. Dixon Arthritis Rheum 2007;56:2905; 8. Suissa Am J Med 2004;117:87; 9. Leombruno Ann Rheum Dis 2009;68:1136. 10. Solomon Ann Rheum Dis 2006;65:1608.
Disclosure:
M. C. Genovese,
Roche Pharmaceuticals,
2,
Roche Pharmaceuticals,
5;
A. Sebba,
Roche Pharmaceuticals, Amgen,
5,
Roche Pharmaceuticals, Amgen, Novartis,
8;
A. Rubbert-Roth,
Roche Pharmaceuticals, Pfizer, Chugai,
2,
Roche Pharmaceuticals, Chugai, UCB, Merck Sharp and Dohme,
8;
J. J. Scali,
None;
R. Alten,
BMS, Novartis, Pfizer, Roche Pharmaceuticals, UCB,
2,
Abbott, BMS, Novartis, Pfizer, Roche Pharmaceuticals, UCB,
5,
Abbott, BMS, Novartis, Pfizer, Roche Pharmaceuticals, UCB,
8;
J. M. Kremer,
Abbott, BMS, Genentech, Janssen, Prizer, UCB,
2,
Abbott, BMS, Genentech,
8;
L. Pitts,
Roche Pharmaceuticals,
3;
E. Vernon,
Roche Pharmaceuticals,
3,
Roche Pharmaceuticals,
1;
R. F. van Vollenhoven,
Abbott, BMS, GlaxoSmithKline, Merck Sharp and Dohme, Pfizer, Roche Pharmaceuticals, UCB ,
2,
Abbott, BMS, GlaxoSmithKline, Merck Sharp and Dohme, Pfizer, Roche Pharmaceuticals, UCB ,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-tocilizumab-in-patients-with-rheumatoid-arthritis-and-a-mean-treatment-duration-of-3-7-years/