Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab is a first-line treatment for remission induction and the prevention of relapse in ANCA-associated vasculitis (AAV). There is a paucity of real-world data on the long-term safety of rituximab in patients with AAV. This study aimed to estimate the incidence of safety events and to compare time to first SAE between a rituximab cohort and a cohort treated with non-rituximab therapy up to 15 years from first exposure.
Methods: RIVAS was a single-center, retrospective observational study including patients with GPA/MPA who had received rituximab (MabThera) or other treatments between 2003 and 2017 at Addenbrooke’s hospital, Cambridge, UK followed up until 30 September 2018. Time 0 was defined as either initiation of MabThera treatment for the rituximab cohort or time of first disease flare/diagnosis for the control cohort. The primary endpoint was time to first SAE. Key secondary endpoints were time to first pre-categorized SAE including serious infection and cardiovascular disorder. Time to second SAE was an exploratory endpoint.
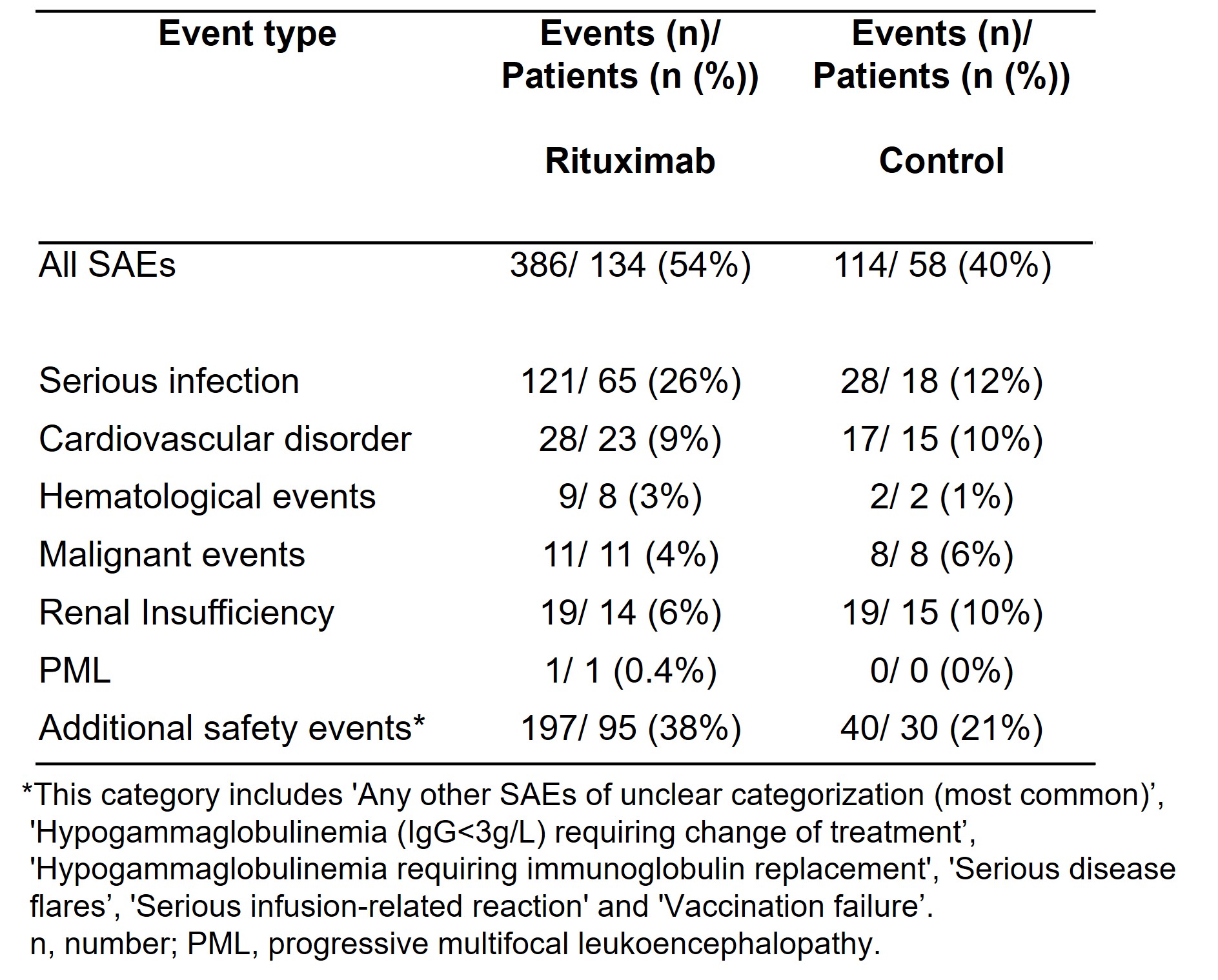
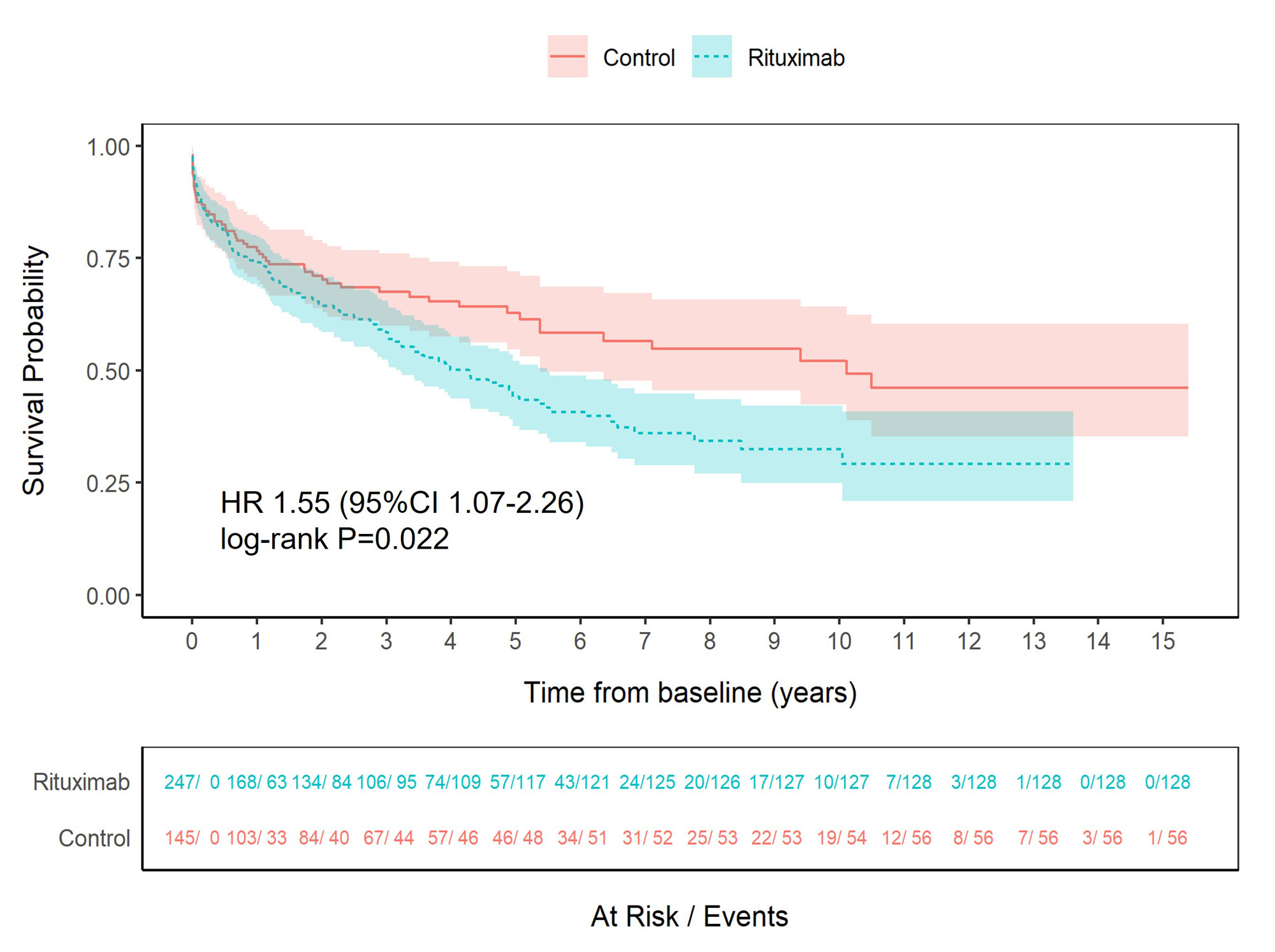
Results: 392 GPA/MPA patients were enrolled: 247 in the rituximab and 145 in the control cohort with a total of 2,217 person-years (mean study duration 5.7 years). Mean age was 61 years (SD 16.3), 52% were female, 77% had GPA and 23% MPA. The median disease duration at baseline was 25.0 months (range: 0-393.7) in the rituximab and 1.6 months (range: 0-272.6) in the control arm. There were differences in the baseline characteristics between groups reflecting the predominant use of rituximab for relapsing or refractory disease. Three hundred and eighty-six SAEs occurred in 134 patients (54%) in the rituximab and 114 in 58 patients (40%) in the control groups (Table 1). Sixty-five patients (26%) in the rituximab group and 18 (12%) in the control group experienced serious infections. Twenty-one patients (9%) in the rituximab group developed severe hypogammaglobulinemia (IgG < 3 g/L) requiring change of treatment and 19 (8%) received immunoglobulin replacement for hypogammaglobulinemia compared to 3 (2%) and 1 (0.7%) of patients in the control group. There were no differences in incidence rates for malignancy, cardiovascular events or renal insufficiency between groups. Time to first SAE was shorter in the rituximab group than in the control group (HR 1.55, 95%CI 1.07-2.26, p=0.022) (Figure 1). Time to first serious infection was shorter in the rituximab group (HR 2.34, 95%CI 1.079-5.07, p=0.031), while no between-group differences were found for other SAE categories. Also, a shorter time to second SAE was observed in the rituximab group. Predictors of first SAE were higher vasculitis damage scores and chronic pulmonary or kidney disease. The relative risk of serious infection and additional safety events was higher in the rituximab group (unadjusted relative risk (RR) 2.12, 95%CI 1.31-3.43; RR 1.86, 95%CI 1.30-2.65, respectively).
Conclusion: Over 40% of patients with GPA/MPA experienced at least one SAE in their disease course. Although the risk of first and second SAE was higher in the rituximab group, baseline imbalances, particularly in disease duration and prior immunosuppressive use, due to the study design were a cause of bias and results should be interpreted with caution.
To cite this abstract in AMA style:
Uchida L, Jones D, Smith R, Loechel C, King M, Nodale M, Bond S, Luqmani R, Gray D, Barrett J, Jayne D. Long-term Safety of Rituximab (Mabthera) in Granulomatosis with Polyangiitis (GPA) or Microscopic Polyangiitis (MPA): Rituximab Surveillance Study in VASculitis (RIVAS) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-of-rituximab-mabthera-in-granulomatosis-with-polyangiitis-gpa-or-microscopic-polyangiitis-mpa-rituximab-surveillance-study-in-vasculitis-rivas/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-of-rituximab-mabthera-in-granulomatosis-with-polyangiitis-gpa-or-microscopic-polyangiitis-mpa-rituximab-surveillance-study-in-vasculitis-rivas/