Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: In the SELECT-COMPARE study, the Janus kinase inhibitor, upadacitinib (UPA), demonstrated significant improvement in the signs and symptoms of rheumatoid arthritis (RA) when administered at 15 mg once daily (QD) on background methotrexate (MTX) compared with adalimumab (ADA) plus MTX at Week 12 that were maintained through 72 weeks in patients with prior inadequate response to MTX.1 The objective of this analysis was to assess the long-term safety and efficacy of UPA vs ADA over 3 years in the ongoing long-term extension (LTE).
Methods: Patients receiving background MTX were randomized 2:2:1 to UPA 15 mg QD, placebo (PBO), or ADA 40 mg every other week. Between Weeks 14-26, rescue was mandated for either lack of response (< 20% improvement in tender or swollen joint counts: Weeks 14, 18, 22) or failure to achieve a targeted disease outcome (CDAI low disease activity: Week 26). Patients who completed the 48-week double-blind period could enter an LTE for up to 10 years total. This analysis describes patients through 3 years of treatment. Treatment-emergent adverse events (TEAEs) per 100 patient years (PY), including events of special interest (AESI), were summarized up to 3 years based on exposure to UPA and to ADA. Efficacy was analyzed by original randomized groups. Patients who were rescued or prematurely discontinued study drug were categorized as non-responders for visits after rescue or discontinuation. Descriptive analyses were performed without formal statistical comparisons.
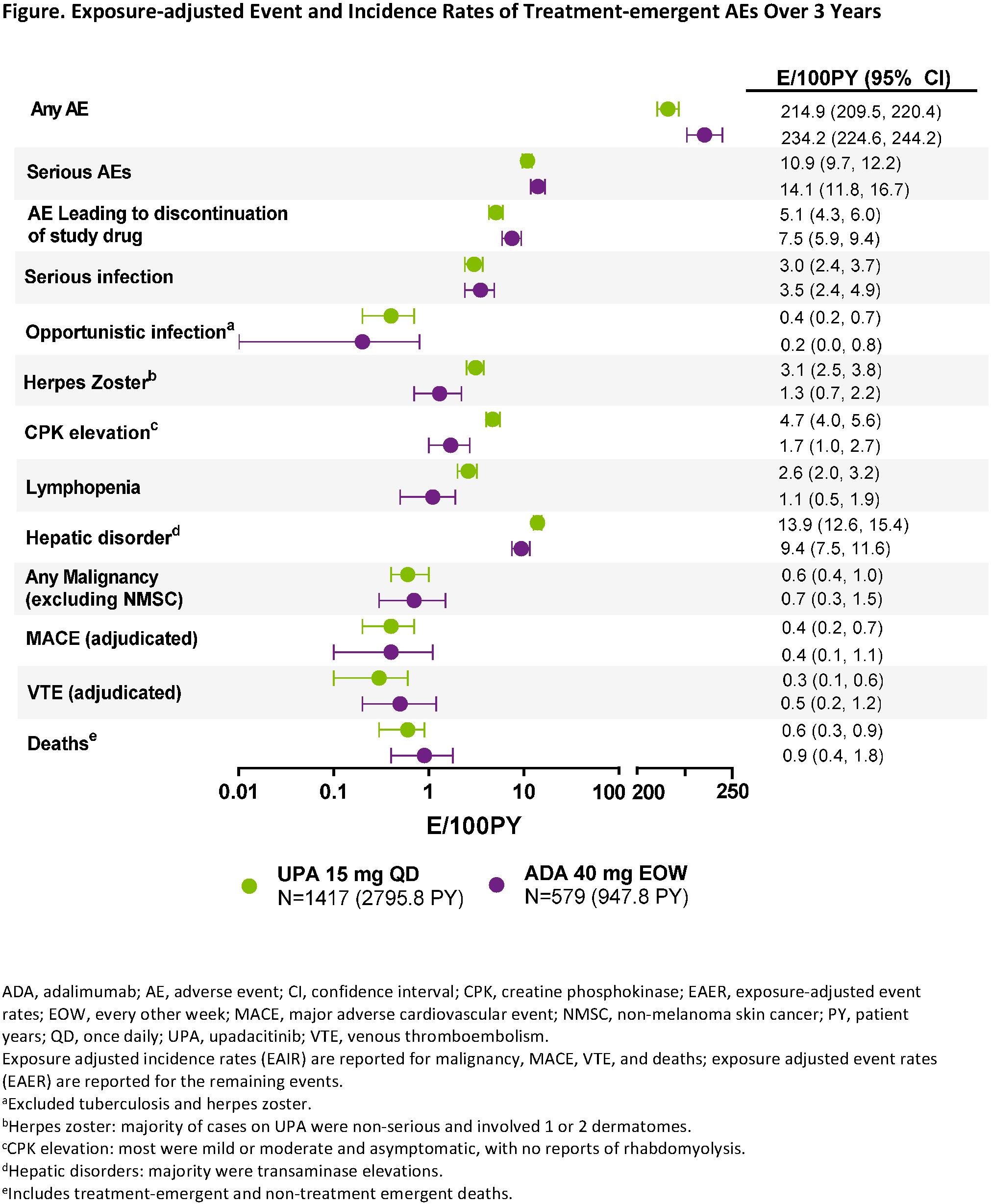
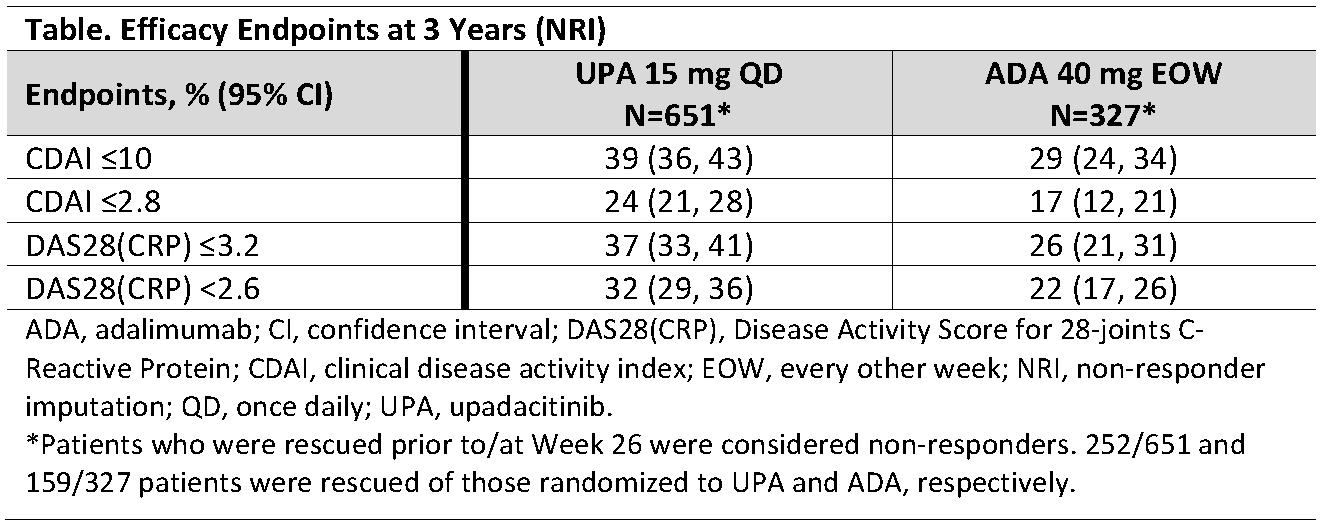
Results: In total, 651, 651, and 327 patients were randomized at baseline to receive UPA, PBO, and ADA, respectively. Between Weeks 14-26, 252 (39%) patients were rescued from UPA to ADA, 159 (49%) were rescued from ADA to UPA, and all PBO patients were switched to UPA by Week 26.1 A higher proportion of patients randomized to UPA completed 3 years without rescue compared to those randomized to ADA (47% vs 36%, respectively). UPA was generally well-tolerated as assessed by the rates of TEAEs, including serious AEs, AEs leading to discontinuation of study drug, and AESIs, including serious and opportunistic infections, malignancies, adjudicated major adverse cardiac events or venous thromboembolism; Figure). Consistent with previous analyses, the event rates of AESIs were generally comparable between the UPA and ADA groups, while herpes zoster, lymphopenia, hepatic disorder, and CPK elevation were reported at higher rates with UPA. Consistent with earlier time points, greater proportions of patients randomized to UPA achieved low disease activity and remission at 3 years based on CDAI, as well as DAS28(CRP) ≤3.2 or < 2.6, compared with patients randomized to ADA (Table).
Conclusion: The safety profile of UPA was consistent with the results reported previously and with the integrated Phase 3 safety analysis.1,2 Higher levels of clinical response continued to be observed with UPA vs ADA through 3 years of treatment.
References:
1. Fleischmann R, et al. Ann Rheum Dis 2020;79:323.
2. Cohen SB, et al. Ann Rheum Dis 2020; doi:10.1136/annrheumdis-2020-218510.
To cite this abstract in AMA style:
Fleischmann R, Mysler E, Bessette L, Peterfy C, DUREZ P, Tanaka Y, Swierkot J, Khan N, Bu X, Li Y, Song I. Long-Term Safety and Efficacy of Upadacitinib or Adalimumab in Patients with Rheumatoid Arthritis: Results at 3 Years from the SELECT-COMPARE Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-upadacitinib-or-adalimumab-in-patients-with-rheumatoid-arthritis-results-at-3-years-from-the-select-compare-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-upadacitinib-or-adalimumab-in-patients-with-rheumatoid-arthritis-results-at-3-years-from-the-select-compare-study/