Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: A proportion of adult patients with RA are refractory to TNF inhibitors (TNFi), and treatment with subsequent biologics may be associated with reduced response. Sarilumab is a human IL-6 receptor inhibitor approved for the treatment of adults with moderate to severely active RA. In the phase 3 TARGET study (NCT01709578), significant improvements in the signs and symptoms of RA and physical function were shown with sarilumab versus placebo in patients refractory to TNFi who were receiving background conventional synthetic DMARDs. Here we investigate the long-term safety and efficacy of subcutaneous sarilumab over 5 years in patients with 1 or >1 TNFi treatment failure prior to their enrollment in TARGET, who continued onto the open-label extension (OLE) study, EXTEND (NCT01146652).
Methods: In the 24-week, randomized, controlled trial TARGET, patients received placebo, sarilumab 150 mg, or sarilumab 200 mg every 2 weeks (q2w), and were eligible to receive open-label sarilumab 200 mg q2w in EXTEND. Dose reduction to 150 mg q2w was permitted per investigator’s discretion, or to manage laboratory abnormalities. Safety outcomes are presented for the entire follow-up period from TARGET baseline through EXTEND. Efficacy was assessed using Clinical Disease Activity Index (CDAI) score. Data cut-off date was January 15, 2019.
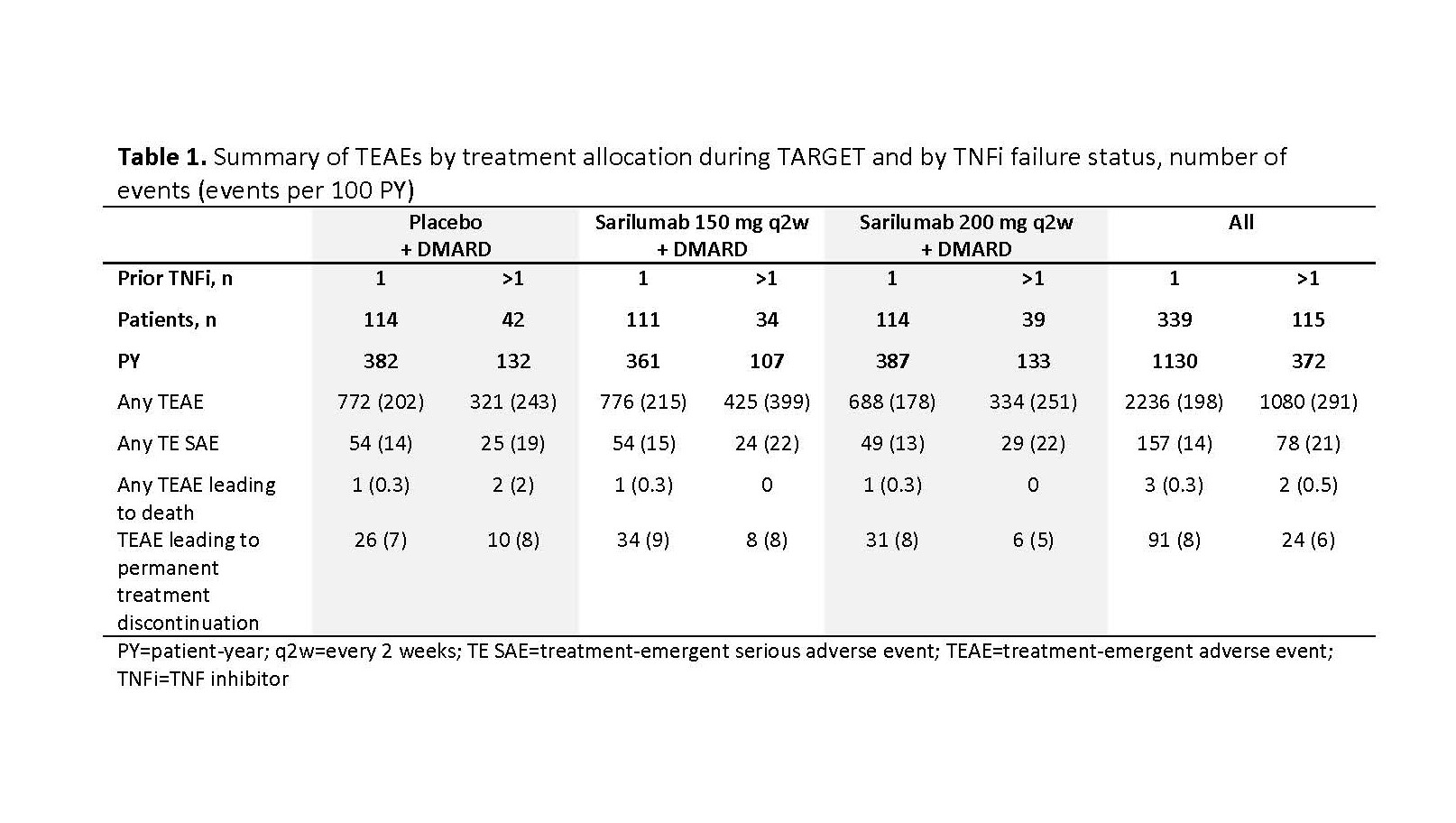
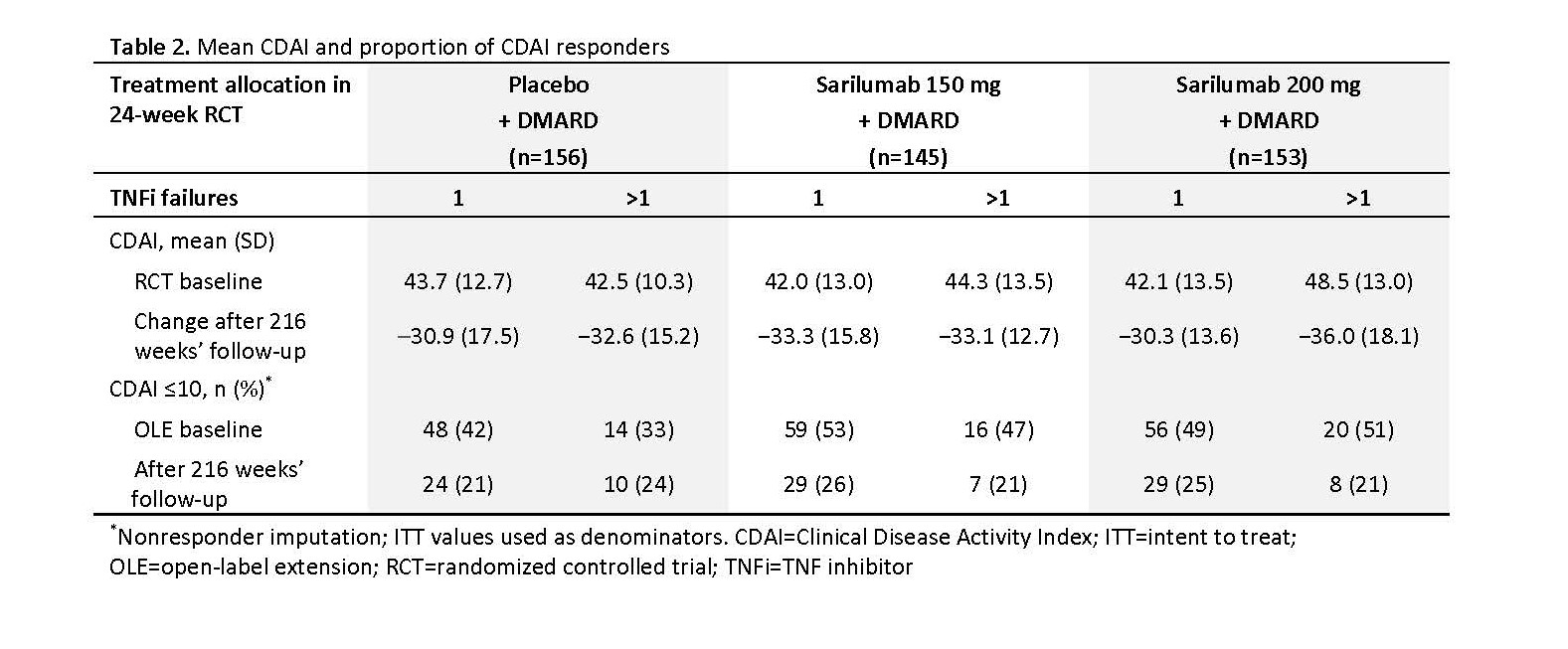
Results: Of the 546 patients randomized in TARGET, 454 (83%) entered EXTEND; of those, 339 had 1 TNFi failure and 115 had >1 TNFi failure. Patients with >1 TNFi failure were older and had a longer duration of RA than patients with 1 TNFi failure (mean ± SD age: 55 ± 13 years vs 52 ± 12 years; RA duration: 14 ± 9 years vs 11 ± 9 years). Kaplan-Meier estimates of the probability of continuation at 5 years were similar between groups: 48% and 54% for patients with >1 and 1 TNFi failure, respectively. By the cut-off date, 36% (199/546) of patients had discontinued treatment during TARGET and EXTEND. In patients with >1 and 1 TNFi failure, there were 291 and 198 treatment-emergent adverse events (AEs) per 100 patient-years (PY), respectively, and 6 and 8 AEs/100 PY leading to discontinuation (Table 1). Clinical efficacy of sarilumab was sustained through 5 years in EXTEND, regardless of initially assigned treatment in TARGET (Table 2).
Conclusion: The long-term safety and efficacy of sarilumab were similar in patients with 1 or >1 prior TNFi failure over 5 years’ follow-up. Clinical efficacy could be sustained through 5 years of treatment.
To cite this abstract in AMA style:
Fleischmann R, Maslova K, Leher H, Praestgaard A, Burmester G. Long-term Safety and Efficacy of Sarilumab over 5 Years in Patients with Rheumatoid Arthritis with 1 or >1 Prior Tumor Necrosis Factor Inhibitor Failures [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-sarilumab-over-5-years-in-patients-with-rheumatoid-arthritis-with-1-or-1-prior-tumor-necrosis-factor-inhibitor-failures/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-sarilumab-over-5-years-in-patients-with-rheumatoid-arthritis-with-1-or-1-prior-tumor-necrosis-factor-inhibitor-failures/