Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody, selectively inhibits IL-17F in addition to IL17A. BKZ has demonstrated clinical efficacy and safety up to 3 years (yrs) in patients (pts) with active AS (i.e., radiographic axial spondyloarthritis)1 in the phase 2b study BE AGILE and its open-label extension (OLE).2
Here, we report 5-yr results of BKZ in BE AGILE and its OLE.
Methods: The dose-ranging BE AGILE study (NCT02963506) consisted of a 12-week (wk) double-blind, placebo-controlled period, then a dose-blind period to Wk 48 where pts received BKZ 160 or 320 mg every 4 wks (Q4W).2 Pts completing Wk 48 were eligible to enter the OLE (NCT03355573) where all pts received BKZ 160 mg Q4W to Wk 256.
Treatment-emergent adverse events (TEAEs; MedDRA v19.0) are reported for BKZ exposure from Wk 0–256. Efficacy is reported from Wk 0–256 for pts enrolled in BE AGILE entering the dose-blind period (dose-blind set [DBS]) and Wk 48–256 for pts enrolled in the OLE with ≥1 efficacy measurement at OLE entry (OLE full analysis set [FAS]). Analyses used non-responder imputation (NRI; pts who did not enter the OLE were considered non-responders from Wk 48), observed case methodology, or multiple imputation (MI).
Results: 296/303 (97.7%) pts randomized in BE AGILE entered the dose-blind period; 265/303 (87.5%) completed to Wk 48. Of 255/303 (84.2%) pts who entered the OLE at Wk 48, and received ≥1 BKZ dose, 202/255 (79.2%) completed to Wk 256.
From Wk 0–256, exposure adjusted incidence rates (EAIRs) per 100 pt-yrs were 134.6 for any TEAE and 5.2 for serious TEAEs (Table). EAIR of Candida infections over 256 wks (2.6) was lower than in Wk 0–48 (7.5).1 All Candida infections were mild or moderate; 1 oral candidiasis event led to discontinuation. No systemic fungal infections were reported. Over 256 wks, EAIRs of serious infections and infestations (1.4) and injection site reactions (0.2) remained low. EAIRs of IBD (0.8) and uveitis (0.7) were also low.
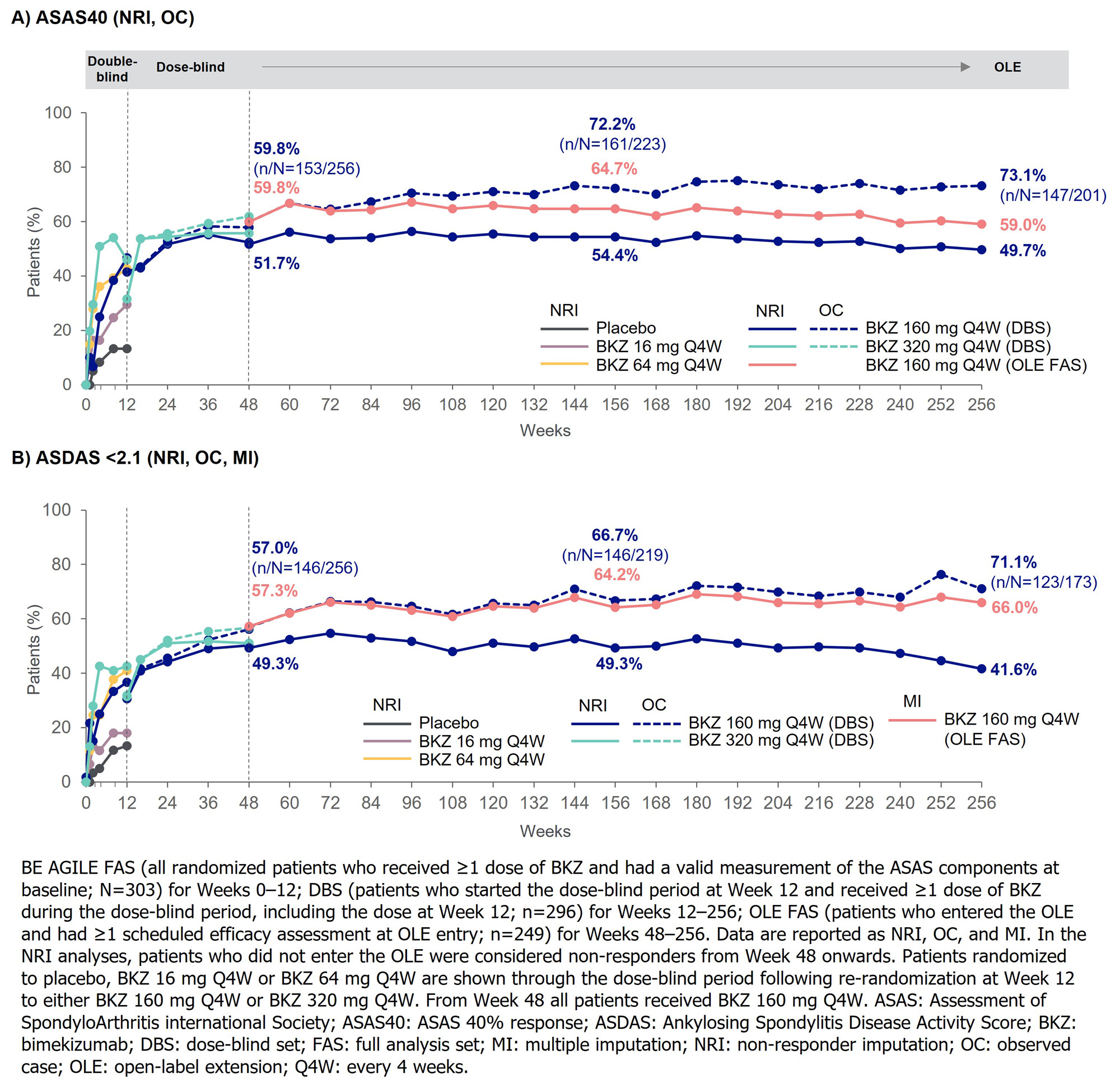
At the OLE entry visit (Wk 48), around half of the DBS (n=296) achieved ASAS40 (51.7%) and ASDAS < 2.1 (49.3%); 49.7% and 41.6% achieved these endpoints at Wk 256, respectively (NRI; Figure 1). Similar results were seen in the OLE FAS (n=249) for ASAS40 (NRI; Wk 48: 59.8%; Wk 256: 59.0%) and ASDAS < 2.1 (MI; 57.3%; 66.0%).
Mean reductions from BL to Wk 48 in ASDAS (3.9 to 2.1) and BASDAI (6.5 to 3.0) in the DBS were sustained or further decreased to 2.1 and 2.5, respectively, at Wk 256 (MI); responses in the OLE FAS from Wk 48 to Wk 256 were similar (MI; ASDAS: 2.0 to 1.9; BASDAI: 2.8 to 2.4).
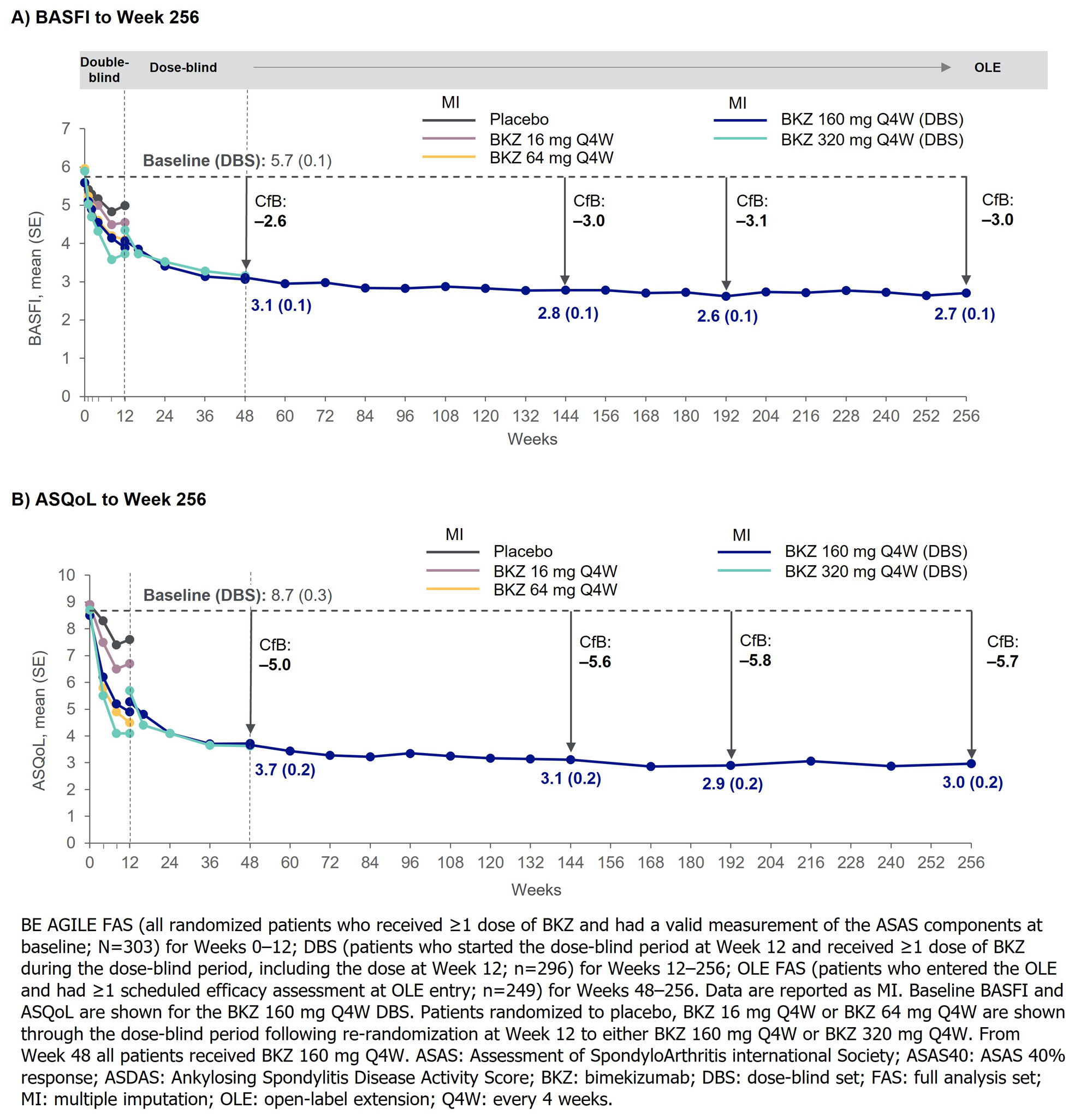
Mean BASFI and ASQoL improvements from BL to Wk 48 were sustained to Wk 256 in the DBS (MI; Figure 2), and from Wk 48 to Wk 256 in the OLE FAS (MI; BASFI: 3.0 to 2.6; ASQoL: 3.3 to 2.6).
Conclusion: The long-term safety profile of BKZ in pts with AS was consistent with previous observations, with no new safety signals identified after 5 yrs of exposure.
Clinical efficacy, including improvements in signs and symptoms, disease activity, physical function, and health-related quality of life, was maintained up to 5 yrs of BKZ treatment. To our knowledge, this is the first report of NRI ASAS40 data up to 5 yrs in pts with AS exposed to novel treatments.
References:1. Boel A. Ann Rheum Dis 2019;78:1545–9; 2. Baraliakos X. Arthritis Rheumatol 2022;74:1943–58.
To cite this abstract in AMA style:
Deodhar A, Navarro-Compán V, Poddubnyy D, Gensler L, Ramiro S, Tomita T, Marzo-Ortega H, Fleurinck C, Vaux T, Massow U, van der Heijde D, Baraliakos X. Long-Term Safety and Efficacy of Bimekizumab in Patients with Active Ankylosing Spondylitis: 5-Year Results from a Phase 2b Study and Its Open-Label Extension [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-bimekizumab-in-patients-with-active-ankylosing-spondylitis-5-year-results-from-a-phase-2b-study-and-its-open-label-extension/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-bimekizumab-in-patients-with-active-ankylosing-spondylitis-5-year-results-from-a-phase-2b-study-and-its-open-label-extension/