Session Information
Date: Monday, November 14, 2022
Title: Abstracts: SLE – Treatment
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Anifrolumab is a fully human IgG1 κ monoclonal antibody that binds to the type I IFN receptor and inhibits type I IFN signaling.1 In the phase 3, randomized, double-blind, placebo-controlled TULIP-1 (NCT02446912)2 and TULIP-2 (NCT02446899)3 trials, demonstration of a favorable benefit–risk profile led to approval of anifrolumab in several countries for patients with moderate to severe SLE receiving standard therapy. Thus, understanding long-term safety and efficacy is essential. Described here are long-term safety and efficacy results of anifrolumab 300 mg vs placebo in patients who completed one year in a phase 3 TULIP trial, plus up to 3 years of participation in the TULIP long-term extension (LTE) study (NCT02794285).
Methods: Eligible patients completed TULIP-1 or TULIP-2 through Week 52 and met all LTE eligibility criteria. In addition to standard therapy, adults received intravenous anifrolumab 300 mg or placebo every 4 weeks for up to 39 doses in the blinded LTE study. Rates of adverse events (AEs), serious AEs (SAEs) including deaths, AEs leading to discontinuation (DAEs), AEs of special interest (AESIs) and laboratory variables were evaluated. Event rates were adjusted for exposure (EAIR) and standardized per 100 patient years. Safety data for patients randomized to anifrolumab 300 mg in the TULIP trials and continuing in the LTE, and those randomized to placebo in all 3 studies, were summarized by descriptive statistics. Exploratory efficacy outcomes included SLEDAI-2K.
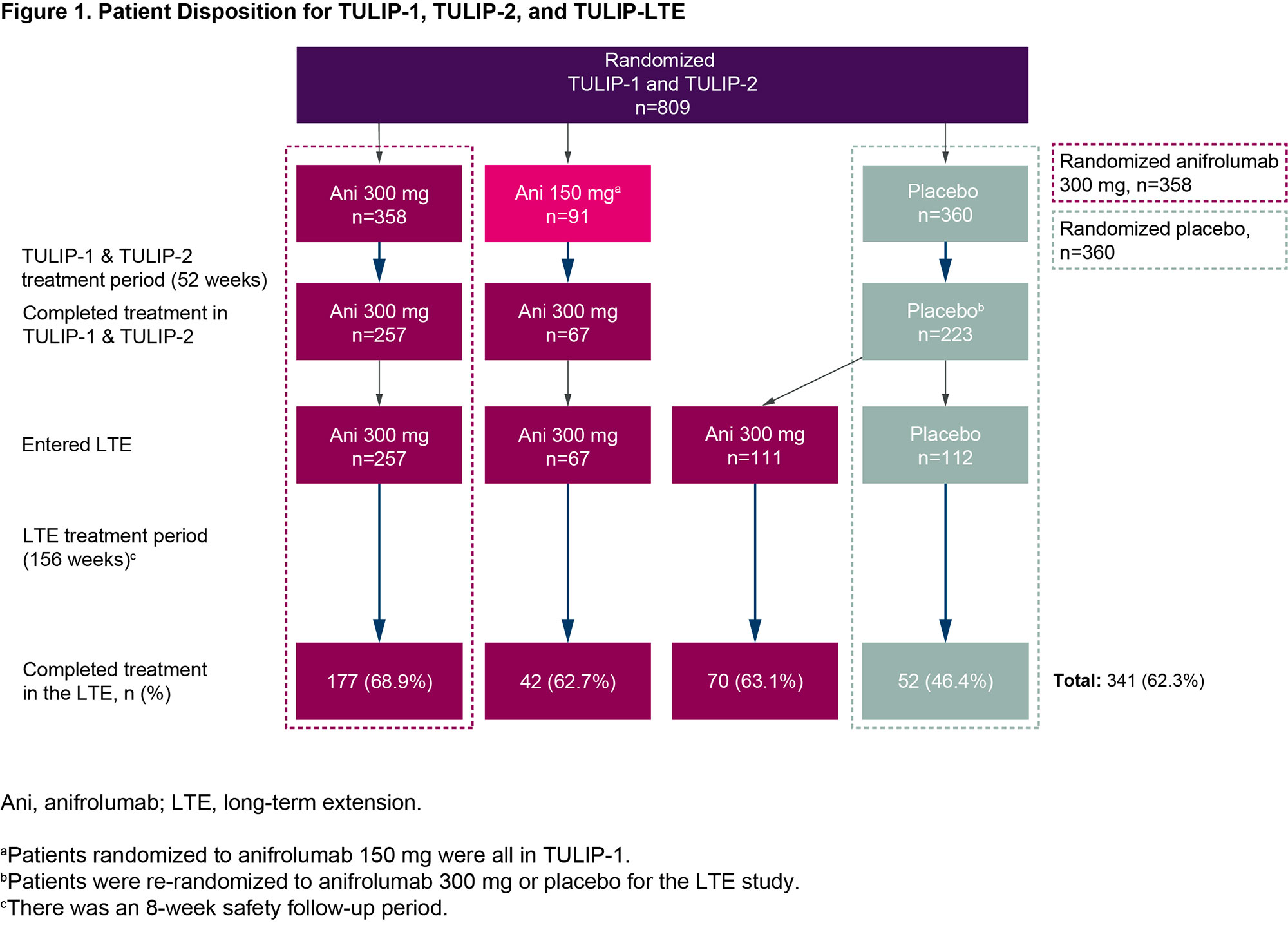
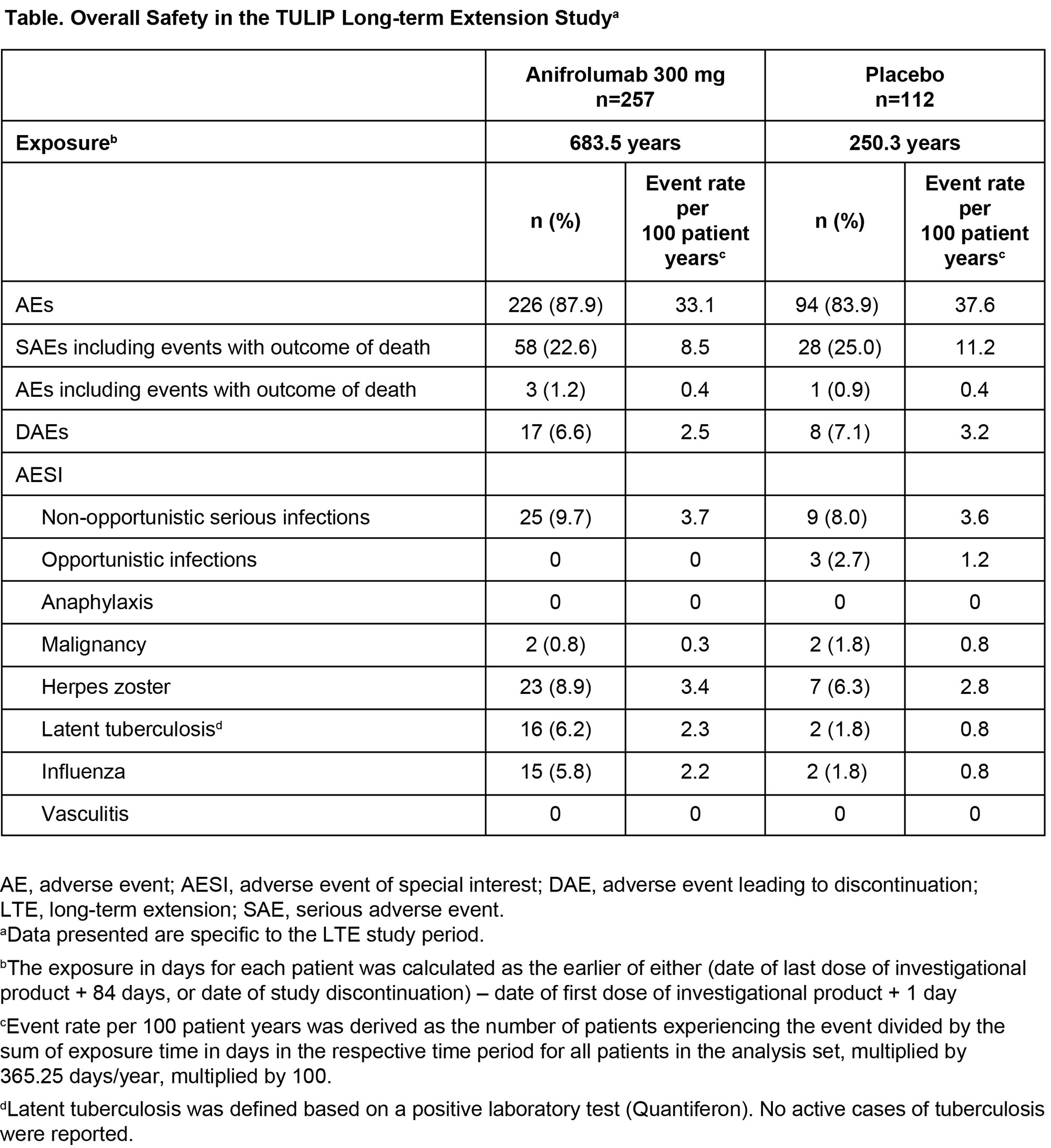
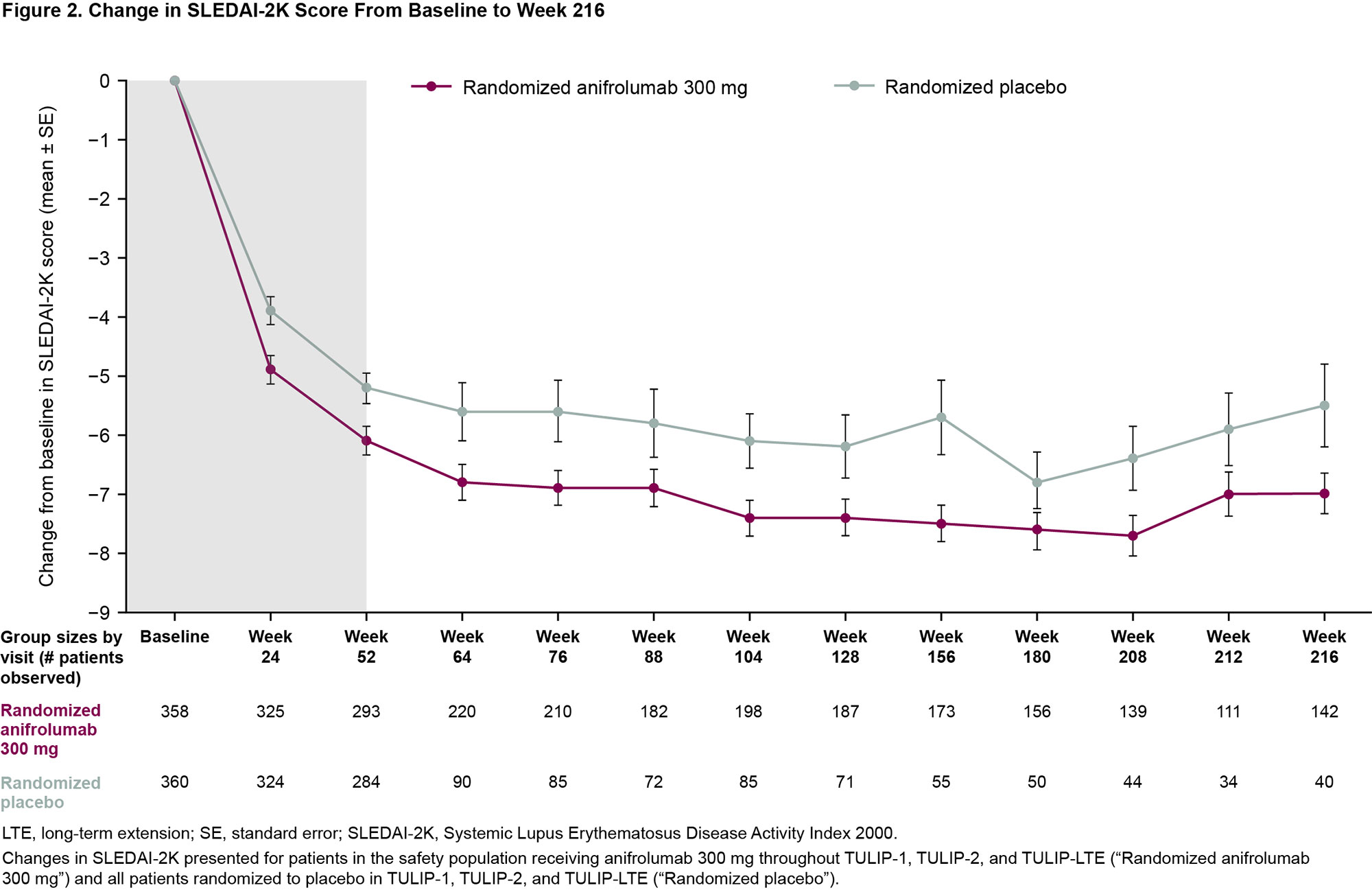
Results: Of 662 patients who completed treatment and the Week 52 visit of TULIP-1 or TULIP-2, 547 enrolled and received at least one treatment dose in the LTE (Figure 1). Along with 257 patients who continued anifrolumab 300 mg, 67 patients transitioned from anifrolumab 150 mg to 300 mg. The 223 patients who received placebo in TULIP-1 or TULIP-2 were re-randomized 1:1 to anifrolumab 300 mg (111 patients) or placebo (112 patients) in the LTE. Treatment was completed by 341 (62%) patients (69% anifrolumab 300 mg, 46% placebo). Frequency and rate of any AEs during the LTE were similar between patients treated with anifrolumab 300 mg or placebo through TULIP-1 and TULIP-2 (Table). EAIRs per 100 patient years of any SAEs, including deaths, were 8.5 for anifrolumab and 11.2 for placebo. EAIRs per 100 patient years were generally similar between groups (anifrolumab vs placebo) for DAEs (2.5 vs 3.2), and for AESI of serious infections (3.7 vs 3.6) and herpes zoster (3.4 vs 2.8). There were no cases of active tuberculosis or anaphylaxis. Rates of malignancy were low, and EAIRs similar, in both groups. Patients who received anifrolumab 300 mg had greater mean improvement in SLEDAI-2K with continued improvement over time (Figure 2).
Conclusion: The favorable benefit–risk profile for anifrolumab compared with placebo in patients with moderate to severe SLE receiving standard therapy was maintained throughout the 3-year LTE of the TULIP trials.
References:
1. Riggs JM. Lupus Sci Med. 2018;5:e000261.
2. Furie RA. Lancet Rheumatol. 2019;1:e208-e19.
3. Morand EF. N Engl J Med. 2020;382:211-21.
To cite this abstract in AMA style:
Kalunian K, Furie R, Morand E, Bruce I, Manzi S, Tanaka Y, Winthrop K, Abreu G, Hupka I, Zhang L, Werther S, Hultquist M, Tummala R, Lindholm C. Long-term Safety and Efficacy of Anifrolumab in Adult Patients with Systemic Lupus Erythematosus: A Multicenter, Randomized, Double-blind, Placebo-controlled 3-year TULIP Extension Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-anifrolumab-in-adult-patients-with-systemic-lupus-erythematosus-a-multicenter-randomized-double-blind-placebo-controlled-3-year-tulip-extension-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-anifrolumab-in-adult-patients-with-systemic-lupus-erythematosus-a-multicenter-randomized-double-blind-placebo-controlled-3-year-tulip-extension-study/