Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Certolizumab pegol (CZP) has been shown to be efficacious and have an acceptable safety profile when administered every 4 weeks (Q4W) as monotherapy (FAST4WARD, NCT00548834) or in combination with methotrexate (MTX) (Study 014, NCT00544154) for the treatment of rheumatoid arthritis (RA).1,2 This post-hoc analysis evaluated long-term safety and efficacy of 400mg CZP Q4W monotherapy and in combination with MTX for RA over 5 years.
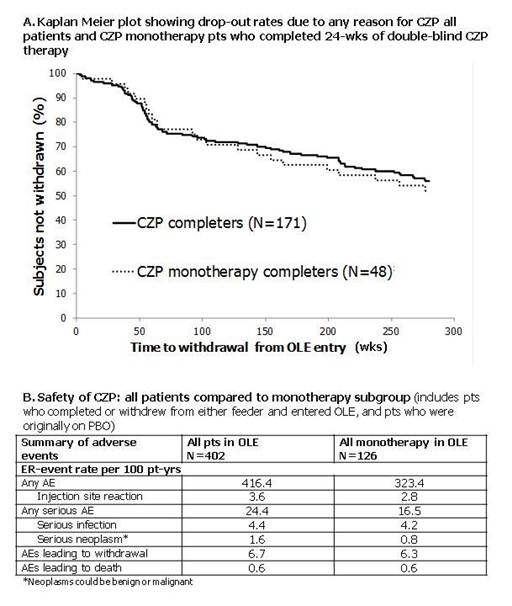
Methods: The open label extension (OLE) study (NCT00160693) enrolled patients (pts) who withdrew or completed the 24 week (Wk) FAST4WARD or 014 studies. The primary OLE objective was to monitor safety. Pts were permitted to take DMARDs in OLE. Pt retention rates and efficacy are reported up to Wk280 (5.4yrs) (last time point all sites open) and safety up to Wk364 (final data cut-point) of the OLE. Safety population included all pts who received CZP in OLE (patients completing or withdrawing early from either feeder study who entered OLE and those originally randomized to PBO in the double-blind phase [all pts N=402, all monotherapy pts N=126]). Analyzed efficacy population consisted of (1) FAST4WARD and 014 Wk 24 completers from CZP group who entered OLE (N=71; CZP completers) and (2) FAST4WARD completers from CZP group who entered OLE and did not receive MTX or another DMARD at any point (N=48; CZP monotherapy completers).
Results: 50% of the CZP monotherapy completers remained in OLE at Wk280 (Figure). These retention rates were similar to all CZP completers (Figure). For all monotherapy pts, the event rates per 100-pt yrs (ERs) for all adverse events (323.4), injection site reactions (2.8), all serious AEs (16.5) and serious neoplasms (0.8) were lower than for all pts (416.4, 3.6, 24.4 and 1.6). ER of serious infections, AEs leading to withdrawal and AEs leading to death were low and similar between populations (Table). In CZP completers and CZP monotherapy completers, respectively, DAS28-3(CRP) mean (SD) change from feeder study baseline was -1.8 (1.2) and -1.9 (1.4) at OLE entry, and -3.0 (1.4) and -3.1 (1.6) at Wk280. HAQ mean (SD) change from feeder baseline for the same groups were -0.4 (0.5) and -0.6 (0.7) at OLE entry, and -0.5 (0.6) and -0.7 (0.8) at Wk280, respectively.
Conclusion: Use of CZP 400mg Q4W monotherapy has been confirmed to have an acceptable long-term safety profile. Some AEs seem to be lower in the monotherapy subgroup than for all pts, but this requires confirmation as it could be a consequence of selection bias. Long-term CZP monotherapy is associated with similar improvement in disease activity and physical function to a population where the majority of pts received combination therapy. CZP monotherapy pts had a similar retention rate in the OLE to the overall population.
References: 1. Fleischmann R et al. Ann Rheum Dis. 2009;68:805-811; 2. Choy E et al. Accepted for publication in Rheumatology November 2011
Disclosure:
R. Fleischmann,
UCB,
2,
UCB,
5;
R. F. van Vollenhoven,
UCB,
2,
UCB,
5;
J. Vencovsky,
UCB,
5,
UCB,
8;
R. Alten,
UCB,
2,
UCB,
5,
UCB,
8;
O. Davies,
UCB,
1,
UCB,
3;
C. Stach,
UCB,
1,
UCB,
3;
M. de Longueville,
UCB,
3;
B. Van Lunen,
UCB,
1,
UCB,
3;
E. Choy,
UCB,
2,
UCB,
5,
UCB,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-4-weekly-certolizumab-pegol-combination-and-monotherapy-in-rheumatoid-arthritis-5-year-results-from-an-open-label-extension-study/