Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Prescription opioids are routinely used in patients with RA. However, use of opioids should be minimized to prevent abuse and addiction. Chronic opioid use doubled from 2002 to 2015 among patients with RA, with severe pain being a strong predictor of use (Lee YC, et al. Arthritis Rheumatol. 2019;71:670-677). This post hoc analysis investigated the association between the effect of treatment with sarilumab, an IL-6 receptor antagonist, and opioid use in patients with RA using clinical trial data.
Methods: Data were collected from placebo-controlled, randomized controlled trials (RCTs) MOBILITY (NCT01061736) and TARGET (NCT01709578) (pooled analysis), and from the adalimumab-controlled RCT MONARCH (NCT02332590). Patients received sarilumab (doses of 150 mg or 200 mg) or placebo once every 2 weeks (q2w) in MOBILITY and TARGET, and sarilumab 200 mg or adalimumab 40 mg q2w in MONARCH. Patients could then enroll in the open-label extension (OLE) studies wherein they received sarilumab 200 mg q2w. Change in concomitant medications, including opioids, was not permitted in the RCTs, but was permitted in the OLEs. Outcomes included evaluation of baseline characteristics associated with higher opioid use, efficacy in both RCTs and OLEs based on opioid use, and the rate of opioid discontinuation in OLEs. Data were analyzed using descriptive statistics and logistic regression.
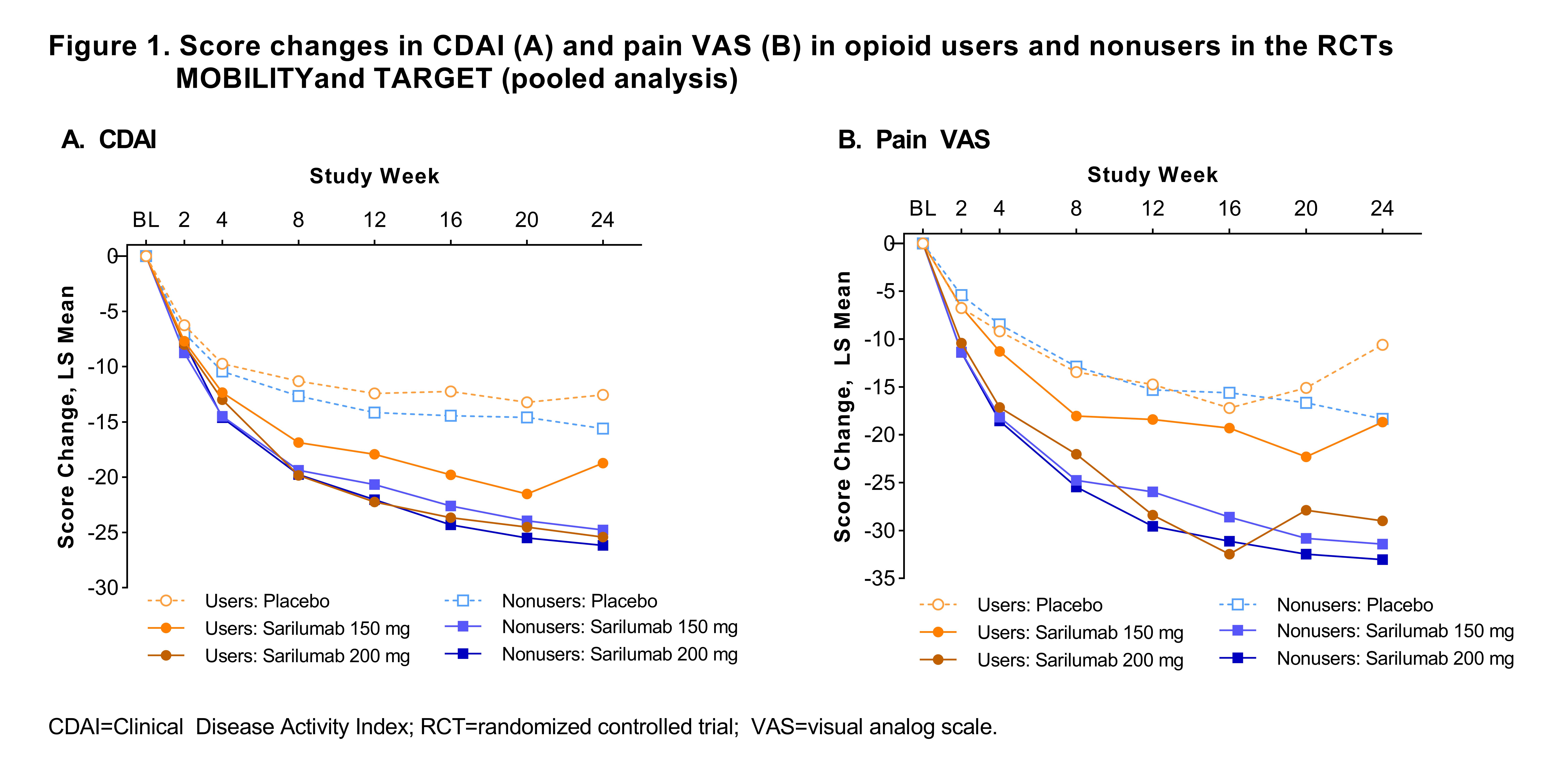
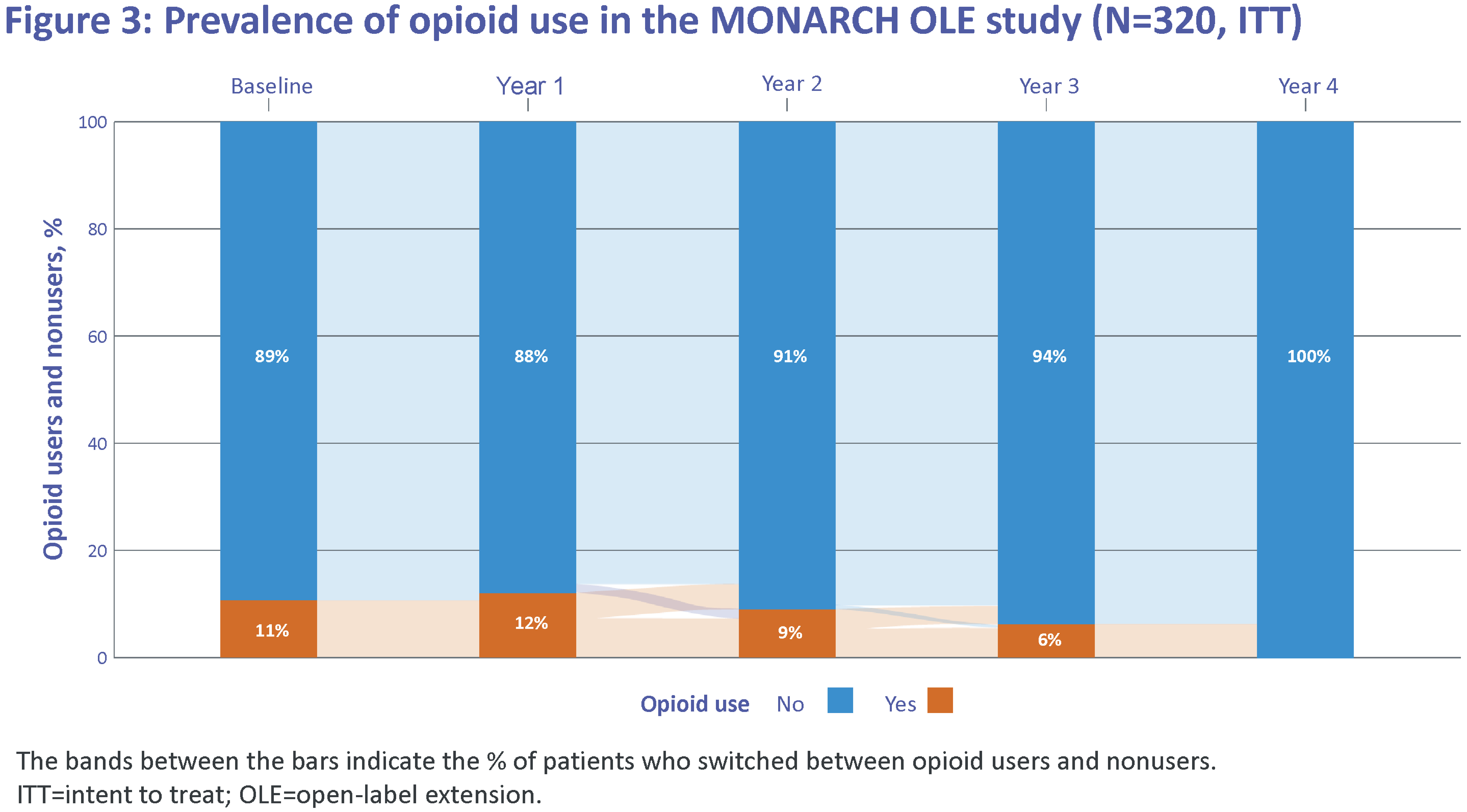
Results: At baseline, 13% (234/1743) of patients in MOBILITY and TARGET, and 10% (38/369) of patients in MONARCH used opioids. Baseline characteristics were consistent among patients with higher opioid use across all trials. Compared with opioid nonusers, opioid users across all trials had higher mean BMI (MOBILITY-TARGET: 32 vs 28 kg/m2; MONARCH: 30 vs 27 kg/m2), longer mean disease duration (MOBILITY-TARGET: 12 vs 10 years; MONARCH: 9 vs 7 years), and higher mean Clinical Disease Activity Index score (MOBILITY-TARGET: 46 vs 41; MONARCH: 46 vs 43). In MOBILITY and TARGET, in both opioid users and nonusers, the reduction in disease activity and pain was numerically greater in sarilumab-treated patients versus placebo, but opioid users treated with sarilumab 200 mg showed greater improvements than those treated with 150 mg (Figure 1). In MONARCH, sarilumab 200 mg was numerically superior to adalimumab 40 mg in reducing both disease activity and pain, regardless of opioid use. After 4 to 6 years of treatment with sarilumab 200 mg in the OLE, efficacy was maintained in patients who were treated with sarilumab in the RCTs, and improved among patients treated with placebo in the RCTs, with those treated with placebo during the RCTs achieving approximately the same level of disease activity as those treated with sarilumab (data not shown). In MOBILITY-TARGET, the proportion of patients using opioids decreased from 10% at OLE baseline to 4% by Year 6 (Figure 2); in MONARCH, the proportion of opioid users decreased from 11% at OLE baseline to 0 at Year 4 (Figure 3).
Conclusion: In this post hoc analysis of long-term data in patients with RA, the efficacy of sarilumab was maintained in both opioid users and nonusers. In addition, the use of opioids decreased over time in patients treated with sarilumab.
To cite this abstract in AMA style:
Pope J, Praestgaard A, van Hoogstraten H, Iglesias-Rodriguez M, Perrot S, Lee Y, Sebba A, Choy E. Long-term Opioid Use in Patients with Rheumatoid Arthritis Treated with Sarilumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-opioid-use-in-patients-with-rheumatoid-arthritis-treated-with-sarilumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-opioid-use-in-patients-with-rheumatoid-arthritis-treated-with-sarilumab/