Session Information
Date: Tuesday, October 23, 2018
Title: Rheumatoid Arthritis – Treatments Poster III: Biosimilars and New Compounds
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The safety and efficacy of certolizumab pegol (CZP) for treating patients (pts) with rheumatoid arthritis (RA) are well-established in a clinical trial setting.1,2 However, data describing these outcomes in routine clinical practice are needed.
Methods:
This was a prospective, observational, multicenter study conducted in France. Data are reported from 2014–2017. Eligible pts were adults with moderate-to-severe, active RA starting treatment with CZP following inadequate response to DMARDs (incl. MTX). Safety outcomes were measured in the safety set (SS; all pts who received ≥1 dose CZP); efficacy in the full analysis set (FAS; SS pts with no protocol deviations).
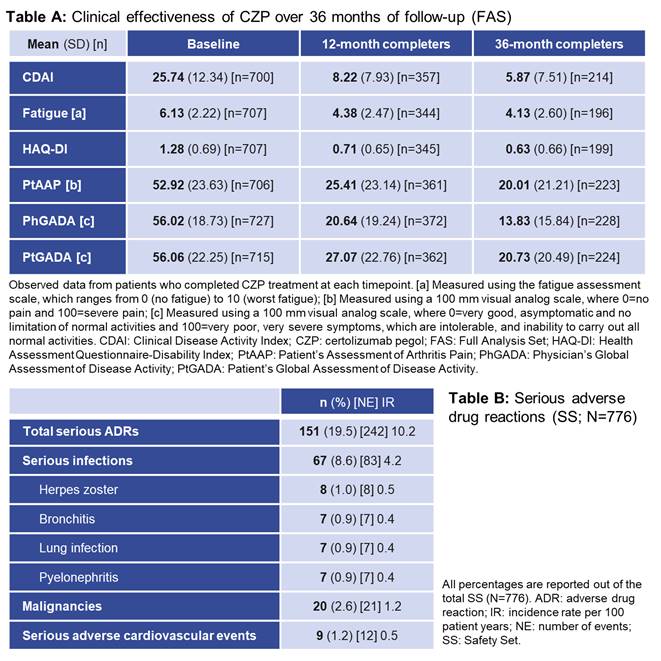
The primary endpoint was EULAR response at the 12-month(mo) visit, and has been reported previously.3 Other measures included clinical disease activity index (CDAI), fatigue assessment scale, health assessment questionnaire-disability index (HAQ-DI), patient’s assessment of arthritis pain (PtAAP), and patient/physician global assessment of disease activity (Pt/PhGADA). Adverse drug reactions (ADRs) and serious ADRs (SADRs) included events with known relationship to CZP, or for which causality was not determined. Data were collected at baseline and routine clinical visits at ~3, 6, 12, 18, 24 and 36mo. Here we report final observed data from 36mo follow-up.
Results:
792 pts were included: 776 (98.0%) in the SS, 733 (92.6%) in the FAS. In the FAS, most pts were female (78.0%), and the mean age at baseline was 55.1 years. Most pts had moderate (n=320, 49.5%) or high (n=265, 41.0%) baseline disease activity based on DAS28(ESR).
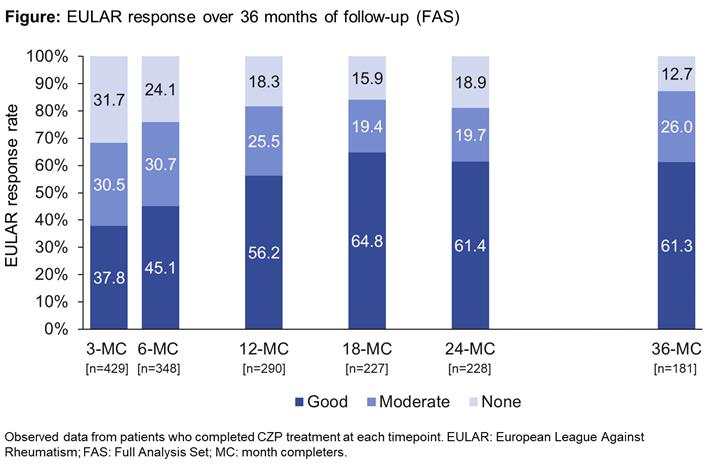
The proportion of pts with a EULAR response increased up to the 12mo visit and was sustained to 36mo (Figure); the proportion of non-responders also remained stable from 12mo. These improvements were reflected in other measures of clinical effectiveness (Table A). Overall, 776 ADRs were reported in 350 (45.1%) pts, including 242 SADRs in 151 (19.5%) pts (Table B). The most frequent ADRs by System Order Class were infections and infestations (268 events in 179 [23.1%] pts). ADRs were consistent with the known safety profile for CZP, with no new safety signals identified.
Conclusion:
RA pts treated with CZP in French clinical practice experienced improvements in disease outcomes after 12mo that were sustained up to 36mo in pts still receiving CZP, with a safety profile that reflects trial data.
References
1. Keystone E. Arthritis Rheum 2008;58:3319–29; 2. Smolen J. Ann Rheum Dis 2009;68:797-804; 3. Saraux A. Ann Rheum Dis 2017;76:786–7.
The study was funded by UCB Pharma, medical writing by Sam Fraser, Costello Medical, UK. We thank the patients who contributed.
To cite this abstract in AMA style:
Saraux A, Flipo RM, Fagnani F, Cukierman G, Bru I, Joubert JM, Schuller JC, Massol J, Combe B. Long-Term Maintenance of Response in Patients with Rheumatoid Arthritis Treated with Certolizumab Pegol [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/long-term-maintenance-of-response-in-patients-with-rheumatoid-arthritis-treated-with-certolizumab-pegol/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-maintenance-of-response-in-patients-with-rheumatoid-arthritis-treated-with-certolizumab-pegol/