Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: There have been increasing reports of cutaneous manifestations during COVID‐19 pandemic. An increase in awareness by physicians made them to increase the number of cryofibrinogen test solicitated. Since some were related to the infectious process, they remained stable or did not reappear during follow-up.
Methods: Unicentric, observational, retrospective study of 116 patients with at least one positive cryofibrinogen determination from December 2022 to December 2023 in a tertiary hospital in Northern Spain. CF syndrome was diagnosed accordingly to reported criteria (two positive test plus cutaneous manifestations or thrombotic events). Asymptomatic patients were defined by not having clinical manifestation. Rest of the patients were included in those who do not meet criteria. During that period, we collected clinical data variables, and laboratory parameters. Most of the patients had at least one more follow-up appointment prior to discharge.
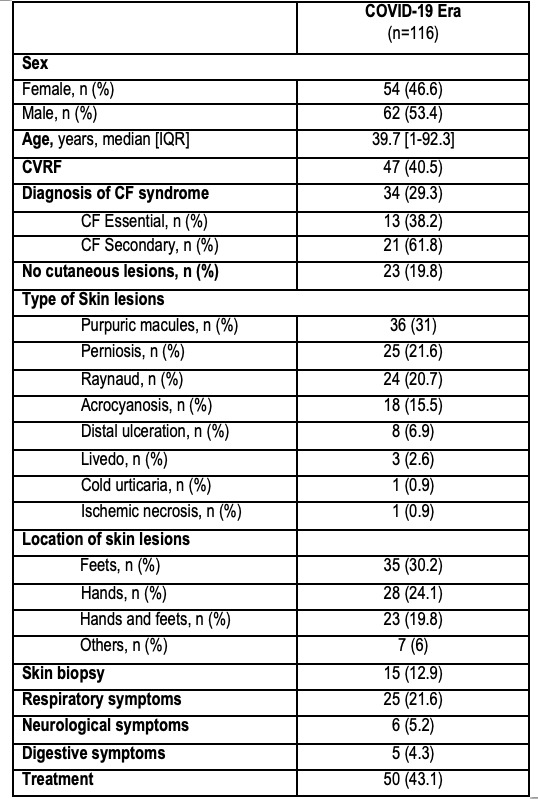
Results: We study 116 patients (53.4% male) (median age 39.7 years old) with positive cryofibrinogen test. Over 40.5% of the patients have any Cardiovascular Risk factors (CVRF). Skin was the most involved organ. Main clinical manifestations related to cryofibrinogenemia included purpuric macules (31%), perniosis (21.6%), Raynaud (20.7%) and acrocyanosis (15.5%). Feets are the most common location, follow by hands. Skin biopsy was performed in only 12.9% of the cases. Overall respiratory symptoms occurred in up to 21% of cases.
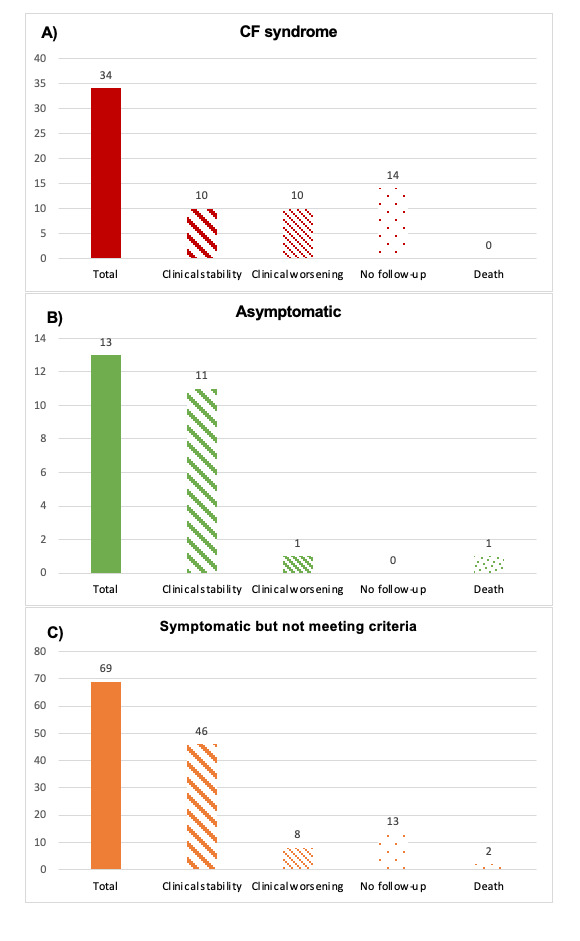
When cryofibrinogen test were performed they were included in these groups a) CF syndrome (n= 34; 29.3%); b) Asymptomatic patients (n= 13; 11.2%); and c) Symptomatic patients who do not meet criteria of CF syndrome (n= 69; 59.5%).
During one year of follow-up (Figure) 10/34 (29.4%) patients with CF syndrome achieved stability and 14/34 (41.2%) patients did not require follow-up. Worsening appeared in 10/34 (29.4%) of them. In the group of asymptomatic patients, 11/13 (84.6%) achieved stability and 1/13 (7.7%) of patients got worse. Finally, 46/69 (66.7%) of patients achieved clinical stability and 13/69 (18.8%) of patients did not require follow up. Worsening was observed in 8/69 patients.
Conclusion: During one year of follow up almost a third of patients with CF syndrome, worsened. By contrast, most patients asymptomatic or symptomatic without meeting criteria of CF syndrome remains stable.
To cite this abstract in AMA style:
Lasa Teja C, Sánchez-López A, Peiró M, Renuncio-García M, Martín-Gutiérrez A, Secada Gómez C, Irure-Ventura J, Lopez-Hoyos M, Blanco-Alonso R. Long-term Follow-up of Patients with Positive Cryofibrinogen Test During the COVID-19 Era [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/long-term-follow-up-of-patients-with-positive-cryofibrinogen-test-during-the-covid-19-era/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-follow-up-of-patients-with-positive-cryofibrinogen-test-during-the-covid-19-era/