Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Biosimilars have enabled some US institutions and payers to achieve significant financial savings after implementing a formulary change from the reference biologic. However, within integrated delivery networks (IDN), health-systems, and outpatient practices, there are short-term administrative costs to clinically implement such a change and additional labor associated with formulary management. The objective of this study is to assess the long-term financial implications of implementing a formulary change from a reference biologic to a biosimilar when considering the short-term costs incurred in select, chronic rheumatologic conditions.
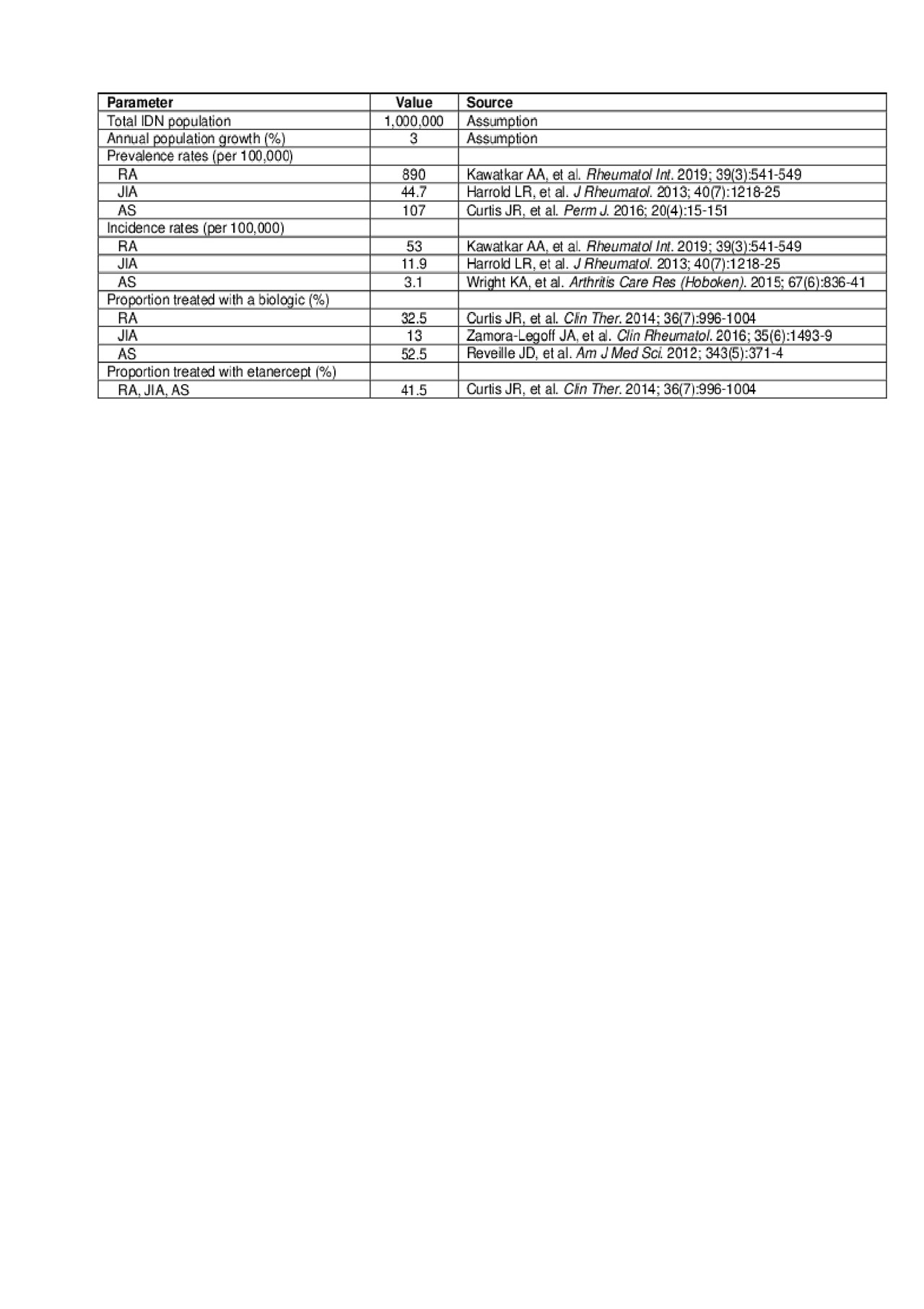
Methods: We evaluated the short- and long-term costs of implementing a formulary change from reference to biosimilar etanercept for the treatment of RA, JIA, and AS from a US IDN perspective over a 5-year time horizon. The number of existing and new patients to be treated with etanercept was calculated based on a hypothetical population of 1 million (M) within an IDN, taking into consideration population growth, etanercept treatment patterns, and incidence/prevalence rates of RA, JIA, and AS derived from the literature (see data table for population inputs). For the price of biosimilar etanercept, a base case discount from reference etanercept was estimated from US market experience with biosimilars in general using Average Sales Price data from the Centers for Medicare and Medicaid Services. For patients switching from reference etanercept, the biosimilar market share was assumed to be 95% in year 1 and 100% by year 5. Initial short-term administrative costs to implement a formulary change vary by institution, so three different scenarios (low: $50,000; medium: $100,000; and high: $200,000) were explored. Labor costs to implement a switch included nurse time for additional patient education and pharmacist time to manage non-formulary requests.
Results: For this hypothetical population of 1 M patients within an IDN, a total of 1,331 (0.1%) patients were treated with etanercept for all three indications by year 5. When considering short-term administrative costs, the total non-pharmacy costs of implementing a formulary conversion program over 5 years was $55,518, $105,518, and $205,518 for the low, medium, and high administrative cost scenarios, respectively. When considering pharmacy cost savings within this particular model, the total 5-year savings were $62.4 M, $62.4 M, and $62.3 M for the low, medium, and high administrative cost scenarios, respectively ($10,076, $10,067, and $10,049 savings per switched patient per year on average).
Conclusion: Substantial cost savings may be realized in the long term for an institution when changing the formulary from the reference etanercept to a biosimilar etanercept, even when factoring the short-term administrative costs and labor associated with managing a formulary change within a relatively small population.
To cite this abstract in AMA style:
Mezzio D, Li E, Balu S. Long-term Financial Impact of Switching from Reference to Biosimilar Etanercept When Considering Short-term Formulary Management Costs in the US [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/long-term-financial-impact-of-switching-from-reference-to-biosimilar-etanercept-when-considering-short-term-formulary-management-costs-in-the-us/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-financial-impact-of-switching-from-reference-to-biosimilar-etanercept-when-considering-short-term-formulary-management-costs-in-the-us/