Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at an increased risk of cardiovascular disease secondary to atherosclerosis. Although it has been reported that TNF inhibitor improved carotid intima-media thickness (IMT) progression, the effect of abatacept (ABT) is unknown. We reported that 24-week ABT treatment was efficacious and safe in both older vs younger patients with RA who were refractory to csDMARDs. Presently, we report the long-term results of ABT on disease activity and safety for 3 years (156 weeks) in the same population while focusing on the effectiveness of ABT on atherosclerosis progression.
Methods: This was a prospective observational study. Patients were stratified by age and treatment into four groups: younger (20-64 years old) and older (≥65 years) patients taking ABT (AY and AO) and csDMARD (CY and CO). Patients could either initiate ABT (ABT group), and those in the csDMARD group could add a csDMARD to their prescribed regimen, or switch to a new csDMARD. Change from baseline in mean IMT of the common carotid artery, IMT max (bulbus, bifurcation, internal and common carotid artery), plaque score, pulse wave velocity (PWV), and EULAR response in the ABT vs csDMARD groups in younger vs older patients were analyzed up to week 156.
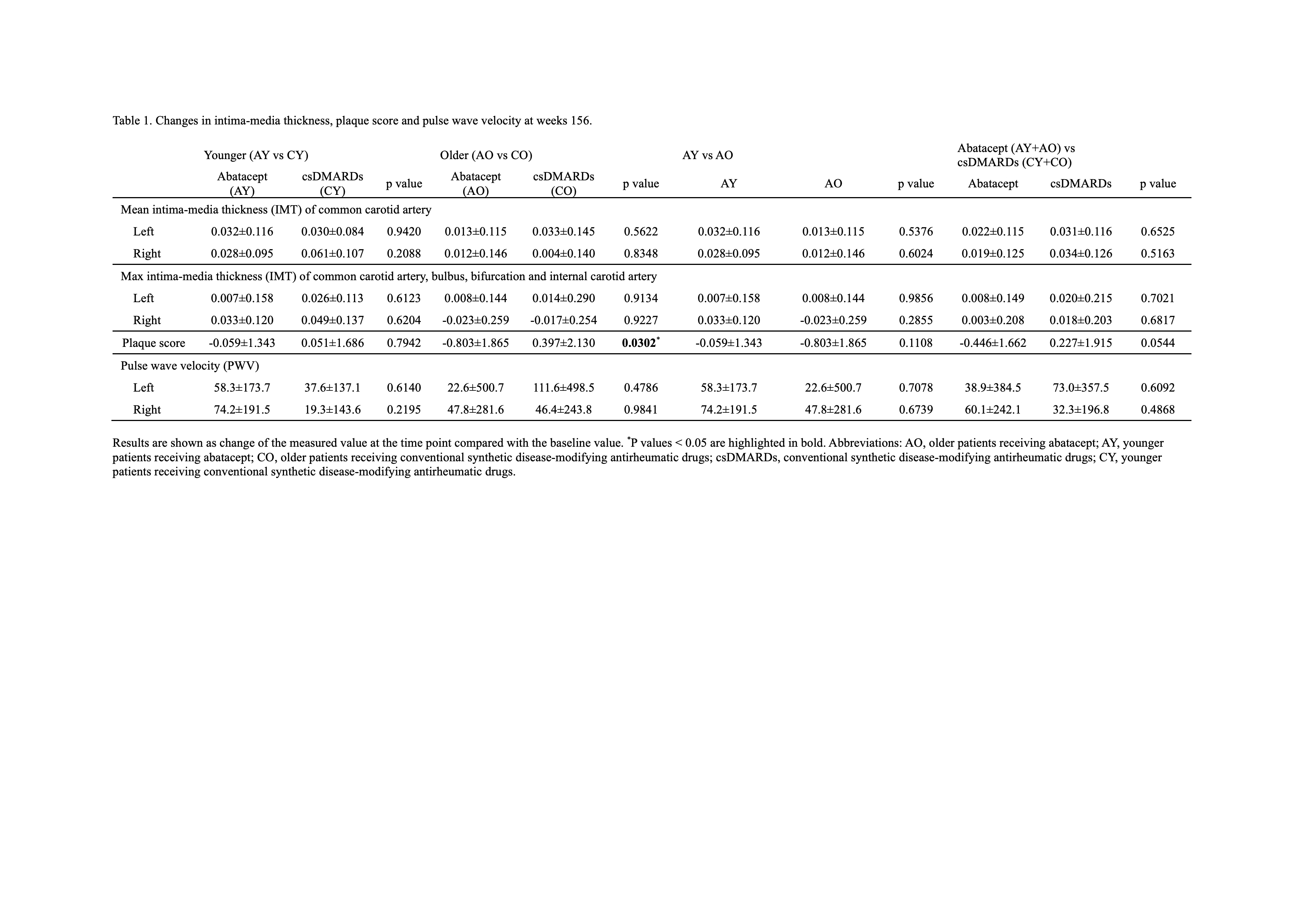
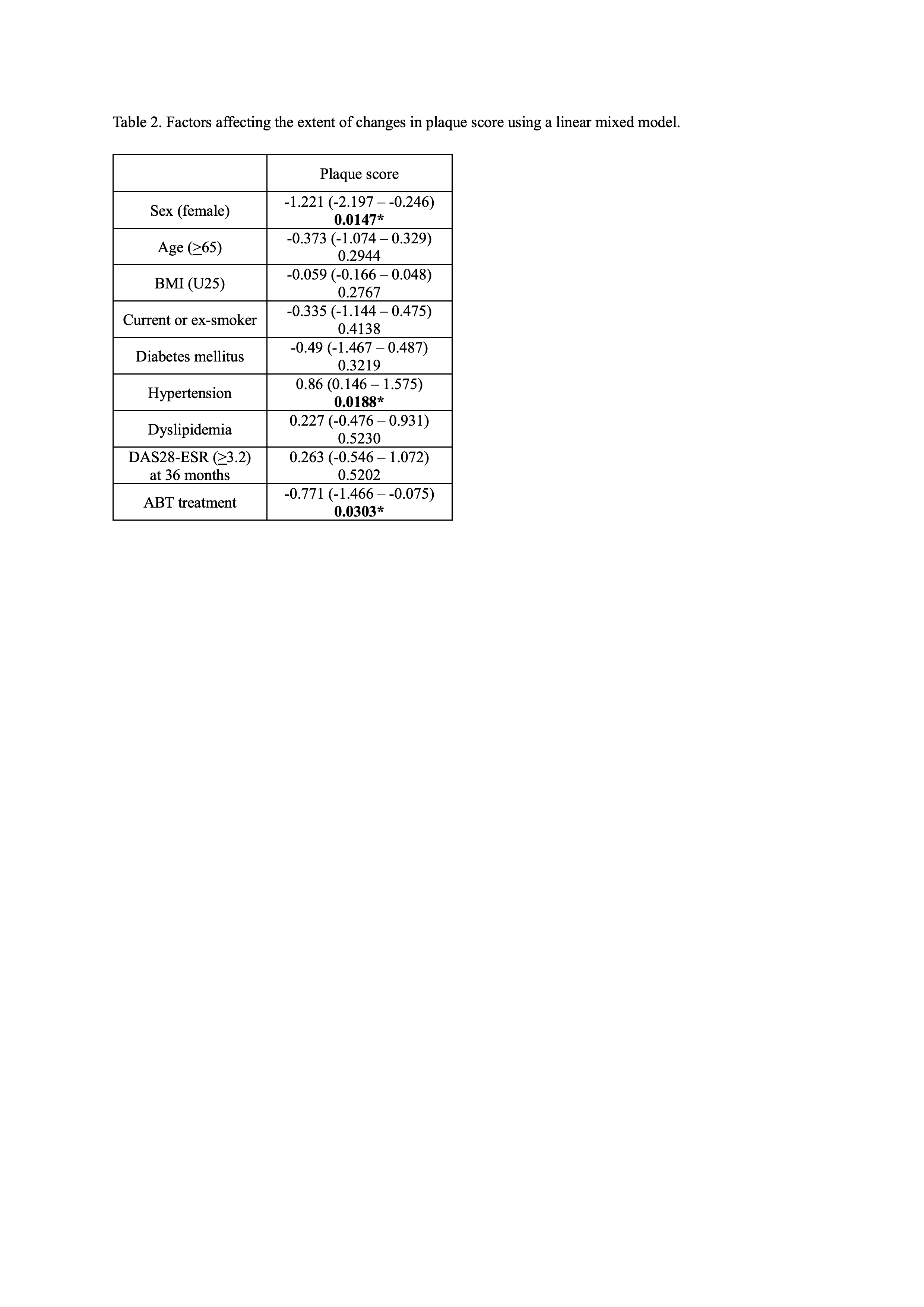
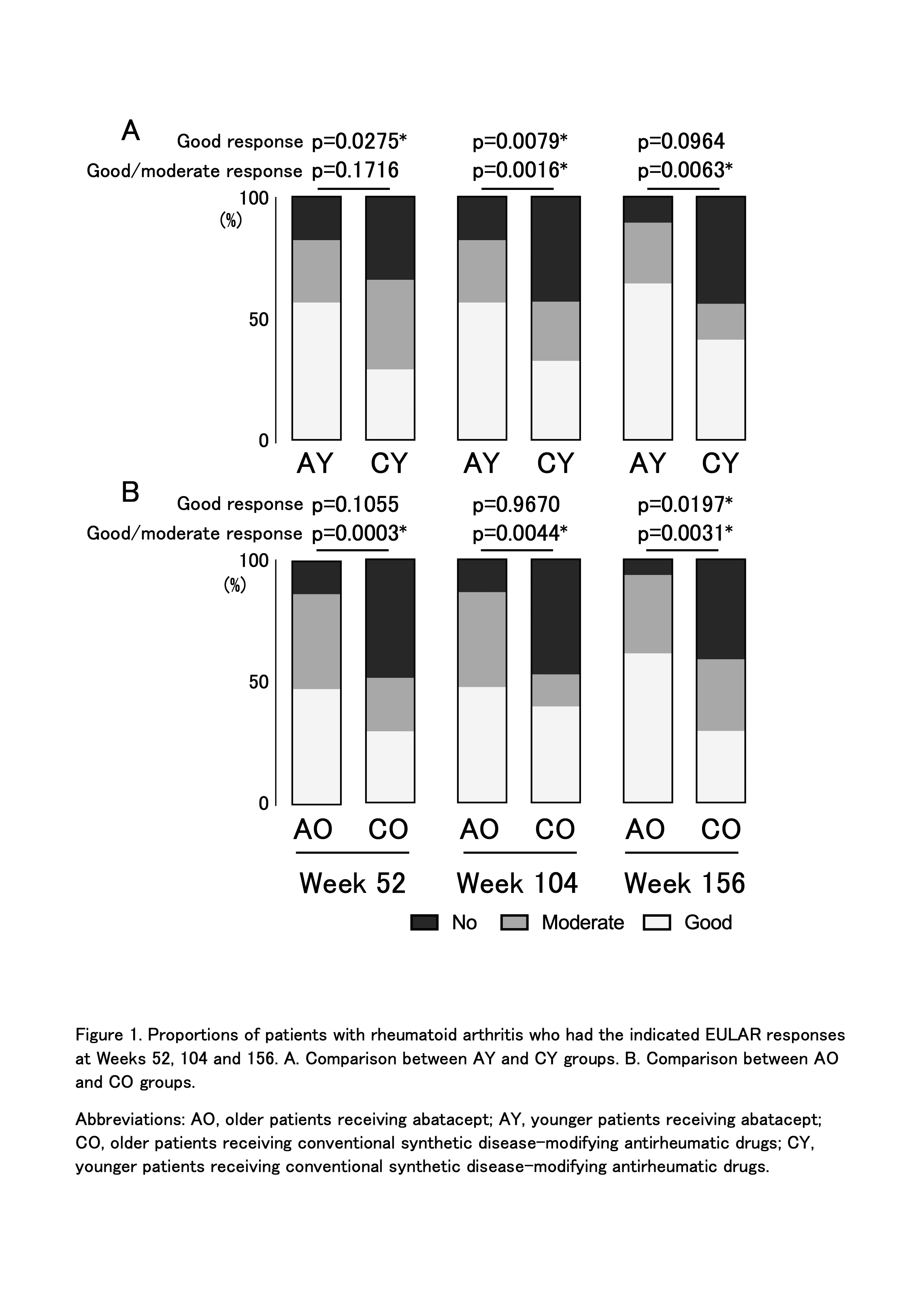
Results: By age and treatment, 216 patients were evaluated (AY, n=52; AO, n=73; CY, n=41; CO, n=50). Change of mean IMT of the common carotid artery and max IMT in the ABT group tended to be small compared with the csDMARD group at week 156 (Table 1). Change of plaque score in the ABT group also tended to be small compared with the csDMARD group at week 156, which was statistically significant in the older patients (p=0.0302). Overall, there was no consistent trend in PWV. Furthermore, multivariate analysis, which also considered disease activity, showed that change in plaque score was significantly smaller in ABT group (AY+AO) compared with csDMARD group (CY+CO) (p=0.0303) (Table 2). In AY vs CY groups, the patient proportions with good or good/moderate EULAR response were higher in the ABT vs csDMARDs group at all time points (Figure 1). In AO vs CO groups, proportions of patients with good or good/moderate EULAR response were also higher in the ABT group. Decrease in DAS28-ESR, simple disease activity index, clinical disease activity index, or HAQ was significantly greater in the ABT vs csDMARDs group in both younger vs older patients. No significant differences in good or good/moderate EULAR response, DAS28-ESR, simple disease activity index, clinical disease activity index, or HAQ were observed between younger vs older patients in ABT-treated groups (AY vs AO). ABT retention rates were not significantly different between younger vs older patients. Adverse events tended to be more frequent in the ABT group, but no significant differences were found.
Conclusion: ABT may be effective in slowing atherosclerosis. ABT efficacy on disease activity was maintained for 3 years without differences in older vs younger patients. No new safety signals were detected.
To cite this abstract in AMA style:
Yamada Z, Muraoka S, Kawazoe M, Hirose W, Kono H, Yasuda S, Sugihara T, Nanki T. Long-Term Effects of Abatacept on Arthritis and Atherosclerosis in Older vs Younger Patients with Rheumatoid Arthritis: 3-year Results of a Prospective, Multicenter, Observational Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-effects-of-abatacept-on-arthritis-and-atherosclerosis-in-older-vs-younger-patients-with-rheumatoid-arthritis-3-year-results-of-a-prospective-multicenter-observational-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-effects-of-abatacept-on-arthritis-and-atherosclerosis-in-older-vs-younger-patients-with-rheumatoid-arthritis-3-year-results-of-a-prospective-multicenter-observational-study/