Session Information
Date: Monday, November 8, 2021
Title: Abstracts: RA – Treatments II: New Findings in Established Therapies (1442–1445)
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: The optimal rituximab (RTX) dose for the treatment of rheumatoid arthritis remains unclear. RTX treatment of 1000mg per 6 months and 2000mg per 6 months were shown to be similarly efficacious (1). The REDO trial showed comparable 6-month efficacy of continued treatment with 500mg and 200mg compared to 1000mg, though formal non-inferiority could not be established (2). We followed trial participants for up to 4 years in this extension study to confirm effectiveness of ultra-low doses.
Methods: Patients from the REDO trial were invited to participate in this study. Treatment decisions were left at the discretion of the rheumatologist and patient. Disease activity (DAS28-CRP), and medication use (b/tsDMARD, csDMARD, gluccocorticoids [GC]) were collected from start of the trial to censoring in April 2021. The primary outcome was disease activity, secondary outcomes were RTX persistence, RTX doses and intervals, and use of comedication.
Disease activity was analyzed using a longitudinal mixed model with random intercepts to account for intra-patient correlations, in two ways: 1. By original randomization and stratification factors (RF/ACPA and csDMARD use). 2. By time-varying total RTX dose received in the year preceding each disease activity measurement, adjusted for current csDMARD or GC use, and RF/ACPA. The original DAS28-CRP non-inferiority (NI) margin of 0.6 was used.
Results: 118 out of 142 REDO patients were included in current analyses (table 1) Reasons for exclusion were: continuing treatment elsewhere (n=3), no informed consent (n=9) and data yet to be collected in 2 study centers (n=12). Mean follow up was 3.2 years (total of 377 patient-years), with 7 patients switching to another b/tsDMARD (Figure 1) upon which they were censored from disease activity analyses.
Disease activity in both ultra-low dose groups was non-inferior to the 1000mg group, with a mean DAS28-CRP (95% CI) during follow-up of 2.2 (2.0-2.4) in the 1000mg group, 2.2 (2.1-2.4) in the 500mg group and 2.3 (2.2-2.5) in the 200mg group.
Analyzed by received RTX dose, lower RTX dose was significantly associated with a higher DAS28-CRP: 0.15 (95% CI: 0.04-0.26) points higher per 1000mg more RTX (Figure 2). The upper limit for relevant RTX doses was below the prespecified NI margin, excluding a relevant effect of RTX dose on disease activity.
Median (IQR) yearly RTX dose was 978mg (704mg-1425mg). Final RTX dose per infusion was 200mg in 37 patients (31%), 500mg in 47 (40%) and 1000mg in 34 (29%), with a median (IQR) final interval between infusions of 6.0 (5.7-6.5), 6.2 (6.0-7.4) and 6.4 (6.0-9.6) months respectively. The rate of GC injections was 0.38 (95% CI: 0.32-0.44) per patient-year and initiation rate of oral GC was 0.05 (0.03-0.08) per patient-year.
Conclusion: A majority of patients treated with ultra-low dose RTX remained on ultra-low doses for up to 4 years, while disease activity remained low and did not relevantly differ between RTX doses, either according to original randomization or by received dose. Switching to other b/tsDMARDS or use of GC was rarely required.
References:
- Bredemeier M et al. Clin Rheumatol 2015 Oct;34(10):1801-5.
- Verhoef LM et al. Lancet Rheumatol. 2019; 1: e145-e153.
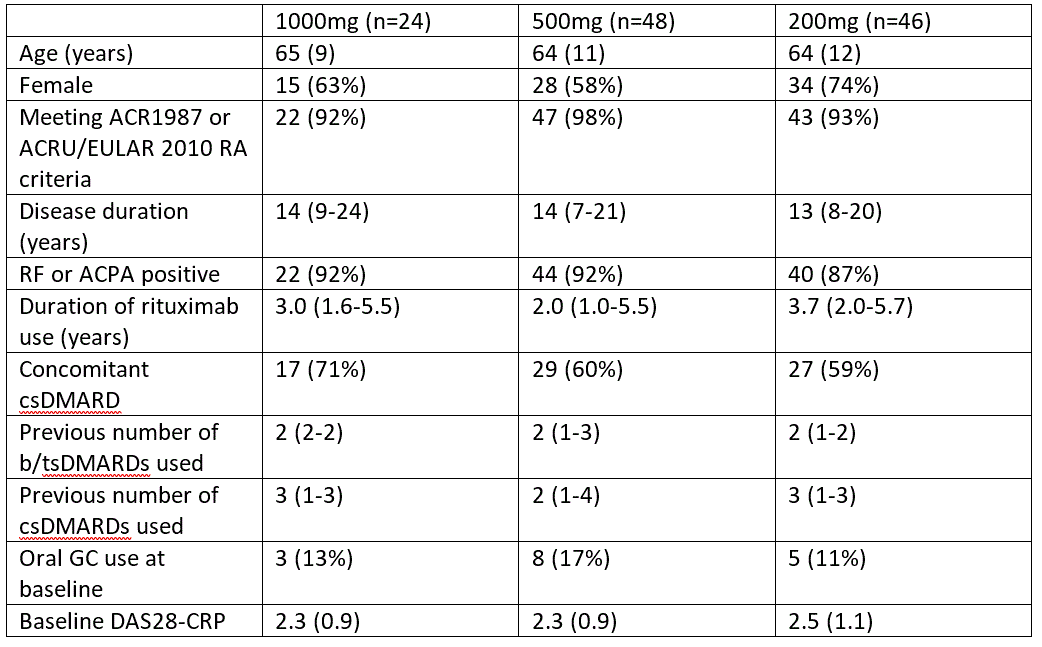 Table 1: Patient characteristics by original randomization. Data are n (%), mean (SD), or median (IQR).
Table 1: Patient characteristics by original randomization. Data are n (%), mean (SD), or median (IQR).
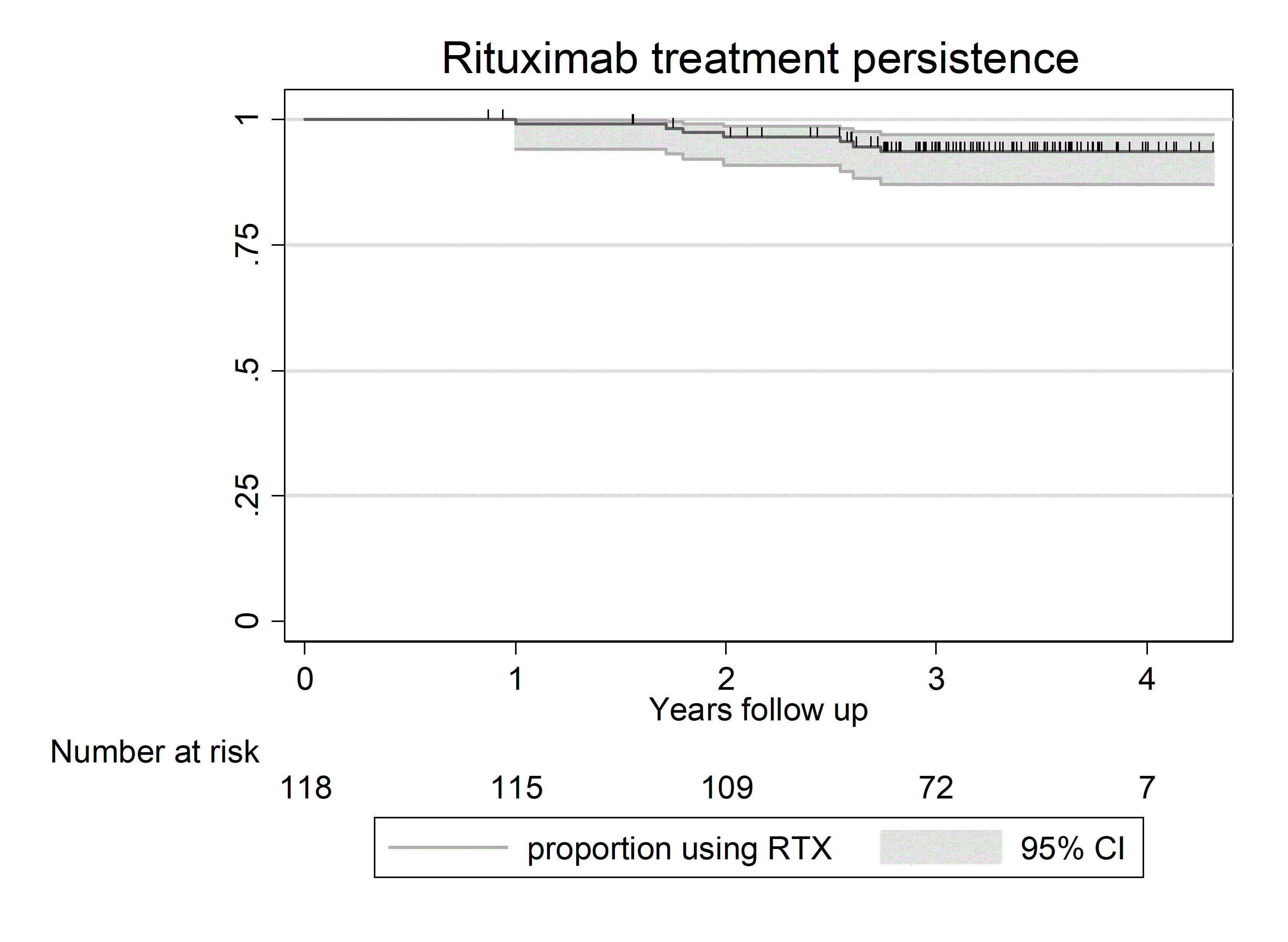 Figure 1: Rituximab treatment persistence during follow up.
Figure 1: Rituximab treatment persistence during follow up.
 Figure 2: Estimated effect on disease activity of RTX dose in the year preceding disease activity measurement, corrected for RF/ACPA, csDMARD, oral and intramuscular glucocorticoid use.
Figure 2: Estimated effect on disease activity of RTX dose in the year preceding disease activity measurement, corrected for RF/ACPA, csDMARD, oral and intramuscular glucocorticoid use.
To cite this abstract in AMA style:
den Broeder N, Verhoef L, De Man Y, Kok M, Thurlings R, van der Weele W, van den Bemt B, van den Hoogen F, van der Maas A, den Broeder A. Long-term Effectiveness of Ultra-Low Doses of Rituximab in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-effectiveness-of-ultra-low-doses-of-rituximab-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-effectiveness-of-ultra-low-doses-of-rituximab-in-rheumatoid-arthritis/
