Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
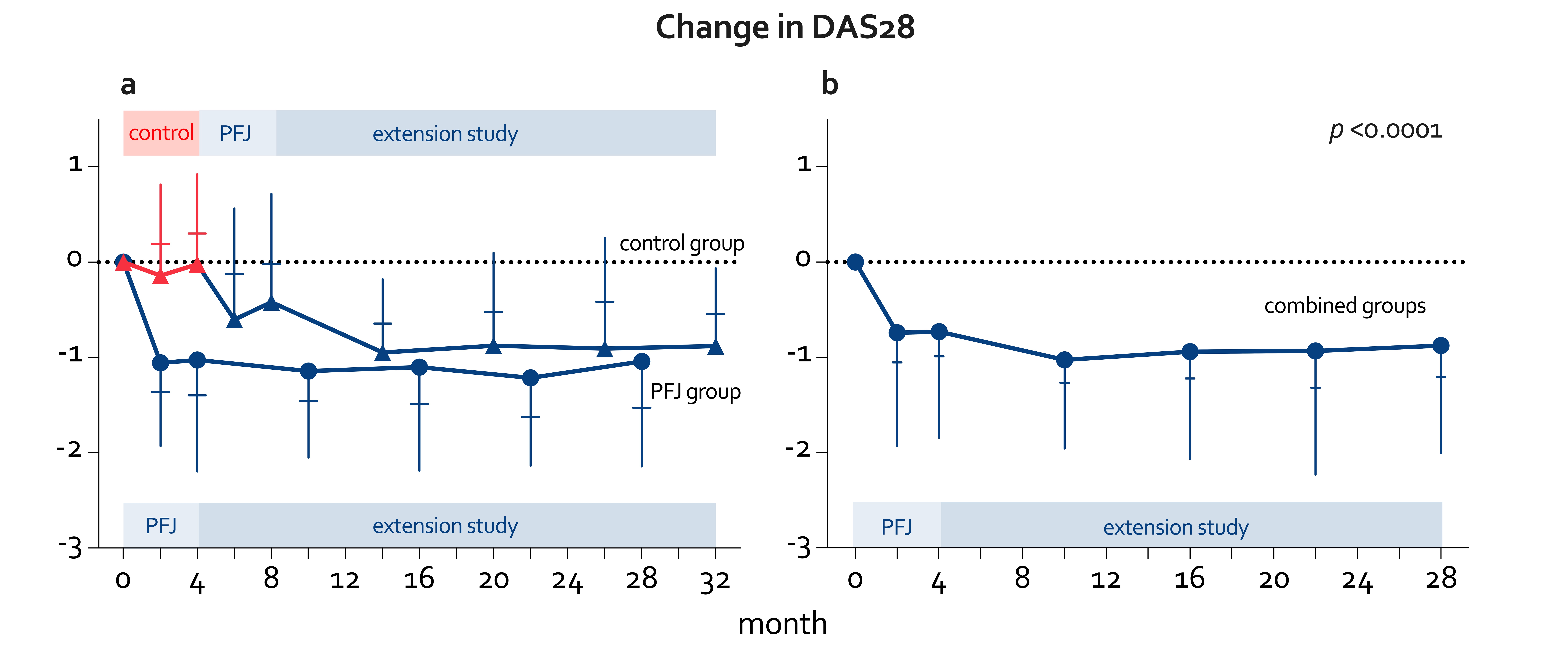
Background/Purpose: The 16-week Plants for Joints (PFJ) multidisciplinary lifestyle intervention, based on a whole-food plant-based diet, physical activity, and stress management, significantly reduced 28-joint Disease Activity Score (DAS28) compared to usual care in people with rheumatoid arthritis (RA).1,2 The aim of this study was to determine the long-term effectiveness of the PFJ lifestyle intervention on disease activity in people with RA two years after the PFJ intervention.
Methods: In the PFJ assessor-blind randomized clinical trial (RCT), people with RA (DAS28 ≥ 2.6 and ≤ 5.1) were randomized to receive the PFJ intervention in addition to usual care, or the control group which received usual care. After this 16-week RCT period the control group also received the intervention. After completion of the intervention participants were followed up for two years with biannual visits and six adherence-promoting webinars per year. Participants with a DAS28 < 2.6 received a protocol as a suggested approach to taper antirheumatic medication with their rheumatologist. Changes in medication intensity between trial initiation and end of the extension study were classified as “increase”, “stable”, or “decrease” by an independent committee. Secondary outcomes included anthropometric and metabolic markers. An intention-to-treat analysis with a linear mixed model was used to analyze changes over time.
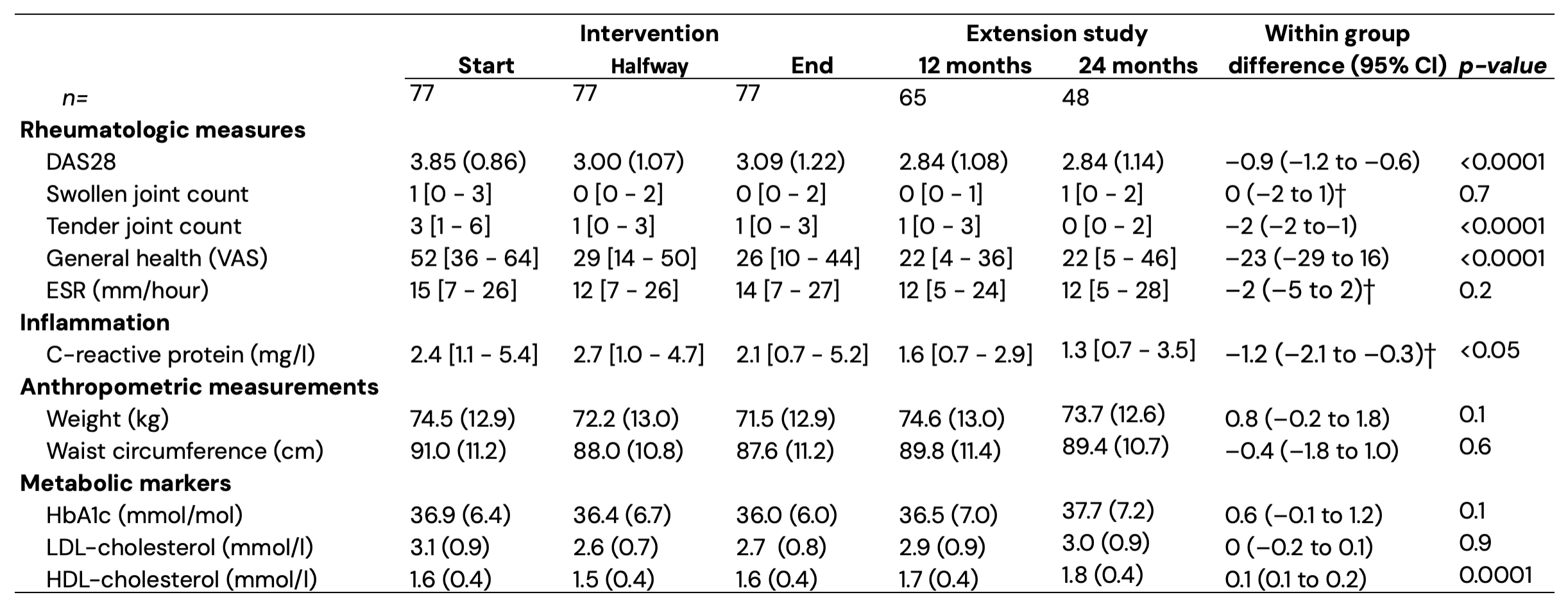
Results: 48 (62%) of the 77 trial completers also completed the two-year follow-up. Overall, 92% of participants were female with a mean (SD) age of 55 (12) and baseline body mass index of 26 (4) kg/m². Two years after completing the PFJ intervention the improvement in DAS28 after PFJ was maintained, and significantly lower compared to baseline: mean –0.9 (95% CI –1.2, –0.6; p < 0.0001; Figure 1). Tender joint count and general health components of the DAS28 improved significantly, while there was no significant difference in the (already low) erythrocyte sedimentation rate and swollen joint count compared to baseline (Table 1). Results were similar in participants completing the two-year extension study vs. those that discontinued prematurely: mean DAS28 change during intervention completer: –0.9, dropout: –0.6; p = 0.4; mean change up to first year extension study completer: –1.0, dropout: –0.9; p = 0.9. Of the 39 participants who completed the follow-up and used disease modifying anti-rheumatic medication, 17 (44%) decreased or stopped, 10 (26%) had stable, and 12 (31%) had increased medication. 30 participants (65%) had improved DAS28 scores (11 with DAS28 < 2.6) with stable or less medication compared to baseline. After the two-year follow-up period HDL cholesterol was increased and CRP remained significantly lower compared to baseline values, although there was no longer a significant difference in weight, waist circumference, LDL cholesterol, or HbA1c.
Conclusion: Significant improvements in disease activity observed after the PFJ intervention were maintained up to two years, while, on average, medication was slightly reduced. These findings indicate that intensive lifestyle modifications can be effective in the long term.
References:
- Walrabenstein, Trials 2021
- Walrabenstein, Rheumatology 2023
To cite this abstract in AMA style:
Wagenaar C, Walrabenstein W, van der Leeden M, Turkstra F, Twisk J, Boers M, van Middendorp H, Weijs P, van Schaardenburg D. Long-term Effectiveness of a Lifestyle Intervention for Rheumatoid Arthritis: Two-year Follow-up After the “Plants for Joints” Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/long-term-effectiveness-of-a-lifestyle-intervention-for-rheumatoid-arthritis-two-year-follow-up-after-the-plants-for-joints-randomized-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-effectiveness-of-a-lifestyle-intervention-for-rheumatoid-arthritis-two-year-follow-up-after-the-plants-for-joints-randomized-clinical-trial/