Session Information
Date: Tuesday, October 23, 2018
Title: Pediatric Rheumatology – Clinical Poster III: Juvenile Idiopathic Arthritis and Uveitis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is a broad term that describes a clinically heterogeneous group of arthritides of unknown cause, which begin before 16 years (yrs) of age and continues into adulthood.1 The purpose of this analysis was to evaluate the long-term safety and effectiveness, including predictors of sustained disease control, of adalimumab (ADA) among patients (pts) with JIA through 6 yrs of treatment.
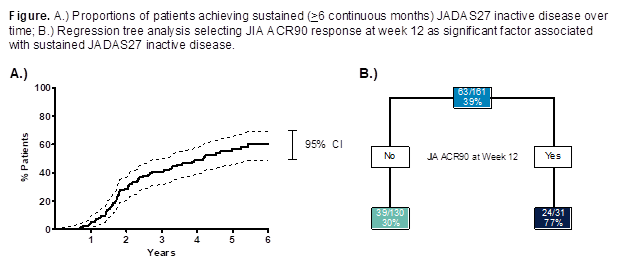
Methods: Children aged 4 – 17 yrs with polyarticular JIA were enrolled in a 32-week (wk), phase 3, randomized-withdrawal, double-blind, placebo (PBO)-controlled trial following a 16-wk open-label (OL) lead-in period. Following completion of the double-blind (DB) period, pts were eligible to enter into a long-term extension (LTE) through 360 wks and receive OL ADA based on body surface area (BSA, 24 mg/m2, maximum of 40 mg eow) for ≥44 wks and fixed dosing (FD, <30 kg: 20 mg eow; ≥30 kg: 40 mg eow) thereafter. Pts were stratified by baseline (BL) MTX use. Adverse events (AEs) were monitored throughout study duration and 70 days beyond last dose. Effectiveness assessments by visit included JIA ACR30/70/90 responses, and the proportions of pts achieving JADAS27 low disease activity (LDA, 1.1 – 3.8) and inactive disease (ID, ≤1). Pts achieving sustained LDA and ID for ≥6 continuous months were evaluated. Regression tree analysis was used to identify BL and post-BL factors associated with sustained ID. Data were as observed without imputation.
Results: A total of 171 pts were enrolled, and 133 were randomized to PBO or ADA. Of these pts, 128 completed the DB period and entered the LTE. Sixty-two pts completed the LTE (primary reasons for study discontinuation: lost to follow-up [n=14], withdrawal of consent [n=20], other [n=22]). Pts on average were 11 yrs of age, and three-fourths were female with nearly 4 yrs of active disease (mean JADAS27, 22.5). Twelve serious infections in 11 pts were reported through >500 pt-yrs of ADA exposure. There were no cases of congestive heart failure-related AEs, demyelinating disease, lupus-like syndrome, malignancies, TB, or deaths reported during any period of the study. At wk 312, 19/28 (68%) and 17/30 (57%) of pts achieved JIA ACR90 and JADAS27 ID, respectively; mean JADAS27 was reduced to 2.5. A total of 63 pts (37%) achieved sustained ID, with a median time of 216 wks to reach sustained ID (Figure). BL factors did not consistently predict sustained ID; however, early JIA ACR responses were associated, with ~75% of pts who achieved JIA ACR90 by wk 12 attaining sustained ID.
Conclusion: ADA appeared well-tolerated among children aged 4-17 with polyarticular JIA through up to nearly 7 yrs of exposure. Significant clinical responses, such as JIA ACR90 and JADAS27 ID, were readily achieved among pts who continued in the study. Early clinical response appeared to predict sustained ID.
Reference:
1. Giancane, et al. Rheumatol Ther 2016(3):187–207.
To cite this abstract in AMA style:
Lovell DJ, Ruperto N, Reiff A, Jung L, Jarosova K, Mouy R, Koné-Paut I, Jones OY, Vargova V, Wouters C, Lagunes Galindo I, Mak C, Brunner HI, Martini A. Long-Term Disease Control Among Patients with Juvenile Idiopathic Arthritis Receiving Adalimumab (Humira) Treatment for up to Six Years [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/long-term-disease-control-among-patients-with-juvenile-idiopathic-arthritis-receiving-adalimumab-humira-treatment-for-up-to-six-years/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-disease-control-among-patients-with-juvenile-idiopathic-arthritis-receiving-adalimumab-humira-treatment-for-up-to-six-years/