Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: About 20-40% of SLE patients develop neuropsychiatric SLE (NPSLE). NPSLE manifestations include impaired cognitive function and depression, both of which negatively impact the quality of life and prognosis of affected patients. Since the molecular mechanisms underlying NPSLE have yet to be fully elucidated, current therapies are nonspecific, broadly immunosuppressive, and less than ideal.
Lipocalins play a role in immune cell differentiation, migration, proliferation, and survival. Lipocalin 2 (LCN2) is a multi-functional acute phase protein in this family with important effects on innate and adaptive immunity. Evidence exists for both inflammatory and anti-inflammatory properties of LCN2 in the brain in a variety of neurological diseases. LCN2 has been implicated in neurotoxicity, glial cell regulation, and protection of the CNS from infection. Under inflammatory conditions, LCN2 expression increases in specific regions of the brain that control anxiety, depression, and cognitive function, suggesting that LCN2 may have an important role in mediating responses to inflammation in the same neuroanatomical regions that are affected in NPSLE.
Methods: MRL/lpr mice, a commonly used lupus model, spontaneously develop anti-dsDNA and other anti-nuclear antibodies as well as kidney, skin, and joint disease, thus mirroring human SLE. In addition, this strain exhibits depression-like behavior as well as deficits in cognition (memory). We generated an LCN2 deficient MRL/lpr mouse (LCN2KO) and evaluated the effects of this constitutive gene knockout on systemic/renal, skin, and brain disease. Systemic/renal disease was quantified by monitoring IgG anti-dsDNA and anti-chromatin antibodies and proteinuria, while skin disease was evaluated by a validated score integrating disease severity and the surface area affected. Neuropsychiatric deficits were quantified through well-established neurobehavioral paradigms. Immune cell infiltration was quantified by flow cytometry (brain) or immunofluorescent microscopy (skin).
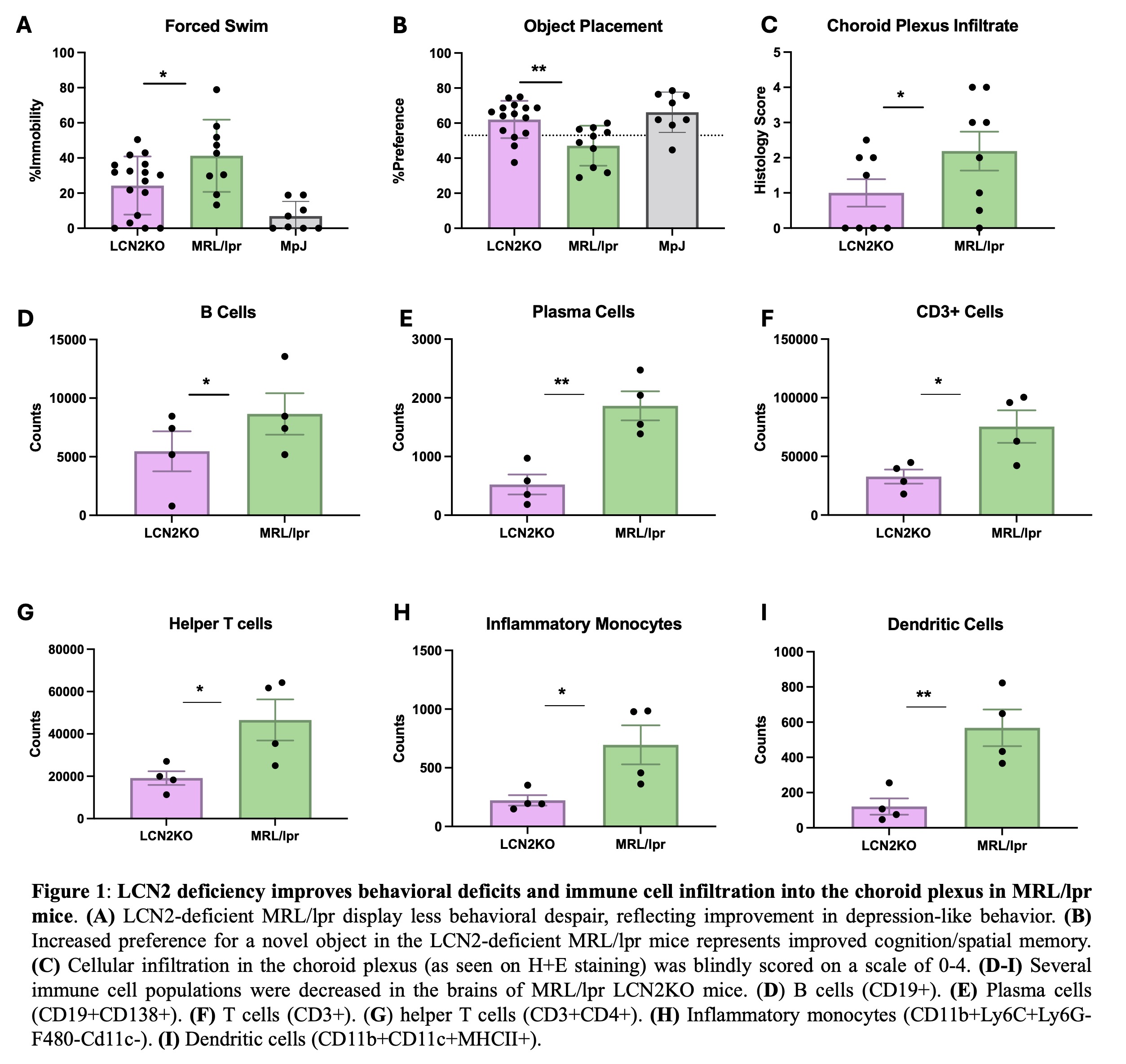
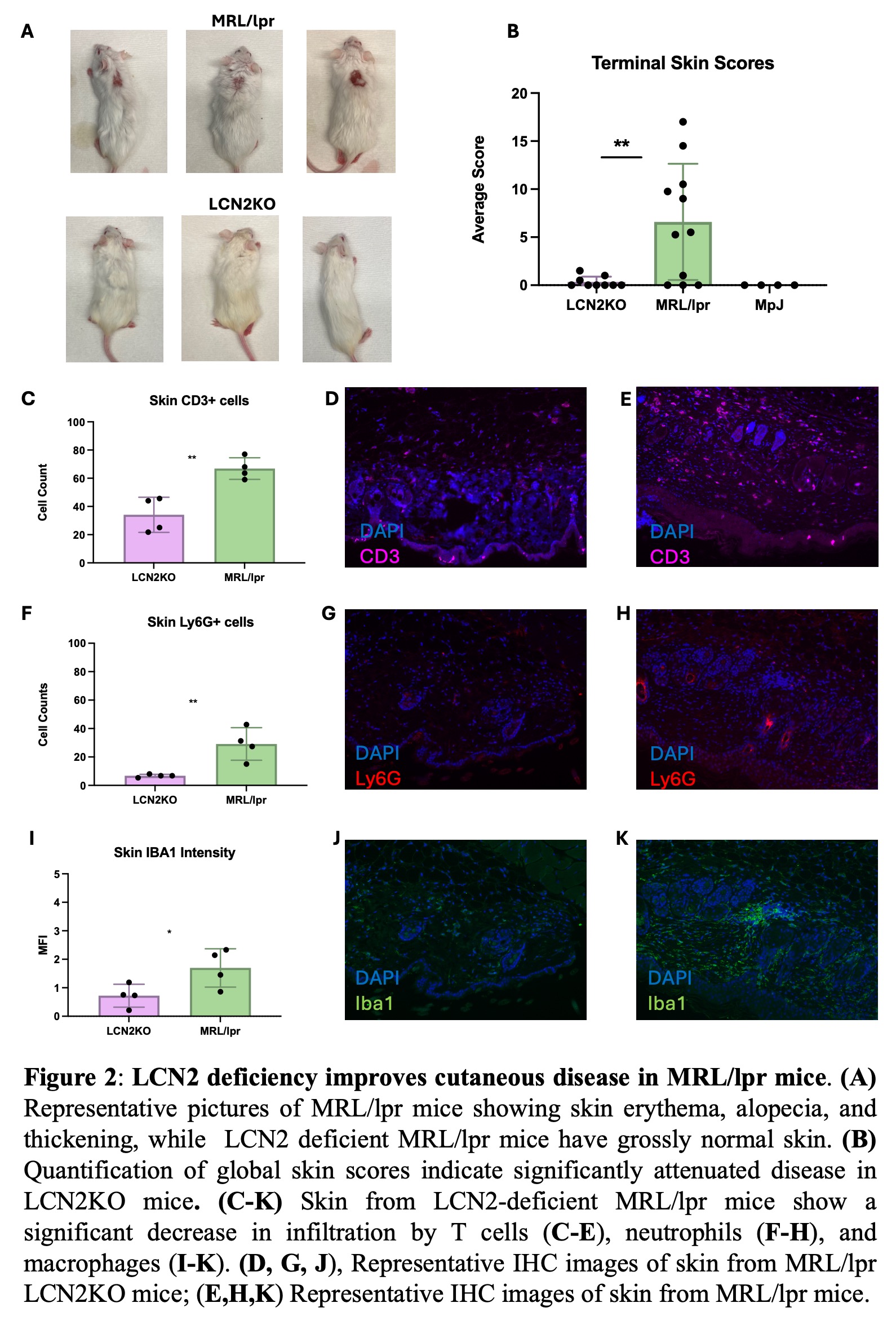
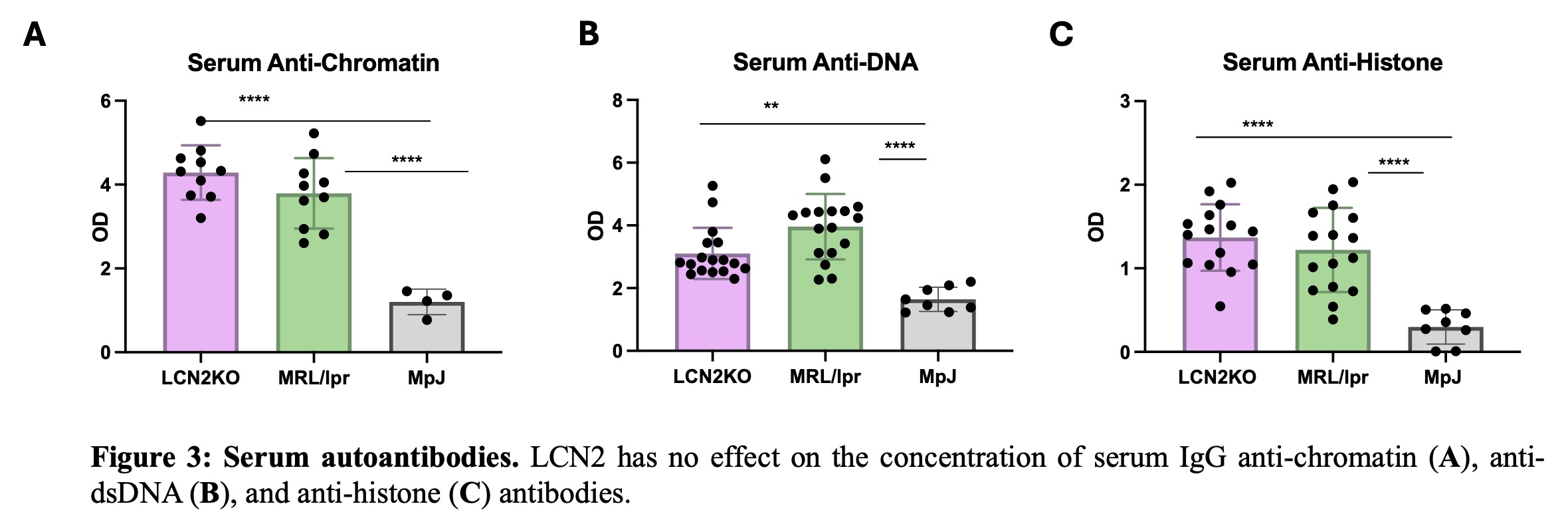
Results: We found that LCN2 deficiency ameliorated spatial memory deficits as well as depression-like behavior in MRL/lpr mice (Fig. 1). We quantified immune cell populations in the brain and found key leukocyte subsets reduced with LCN2 deficiency, suggesting that LCN2 may play a role in the recruitment of particular cell types. Specifically, we found a significant reduction in choroid plexus B cells, CD4+ and CD8+ T cells, dendritic cells, and inflammatory monocytes (Fig. 1). Additionally, LCN2 deficient mice showed significant improvements in cutaneous disease, and immune cell infiltrates were significantly reduced in LCN2 deficient mice (Fig. 2). Interestingly, despite improving cutaneous and neuropsychiatric disease, LCN2 deficiency did not reduce serum anti-nuclear antibodies (Fig. 3) or change the immune cell subset distribution of PBMCs and splenic immune cells, suggesting that LCN2 plays a localized role in target organ injury in SLE.
Conclusion: These data implicate an organ-specific and localized role of LCN2 in SLE, suggesting a potential role for blocking LCN2 as a novel approach for cutaneous and neuropsychiatric disease in SLE.
To cite this abstract in AMA style:
Garcia S, Mike E, Zhang J, Cuda C, Putterman C. Lipocalin-2 Drives Neuropsychiatric and Cutaneous SLE Through Regulation of Immune Cell Recruitment in Target Organs [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/lipocalin-2-drives-neuropsychiatric-and-cutaneous-sle-through-regulation-of-immune-cell-recruitment-in-target-organs/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lipocalin-2-drives-neuropsychiatric-and-cutaneous-sle-through-regulation-of-immune-cell-recruitment-in-target-organs/