Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In patients with active RA an increase in lipid analytes has been observed after treatment with janus kinase inhibitors and other disease-modifying antirheumatic drugs.1 The molecular mechanisms underlying these lipid changes are under active investigation.2
Methods: Data were analyzed from Phase 2 and Phase 3 studies of 4 mg oral baricitinib (bari) administered once daily in patients with moderately to severely active RA. The effect of statin therapy on lipid levels was evaluated in a subgroup of Phase 3 patients. In 1 Phase 3 study, the lipoprotein particle size and number were evaluated with nuclear magnetic resonance (NMR) at baseline and Week (Wk) 12.
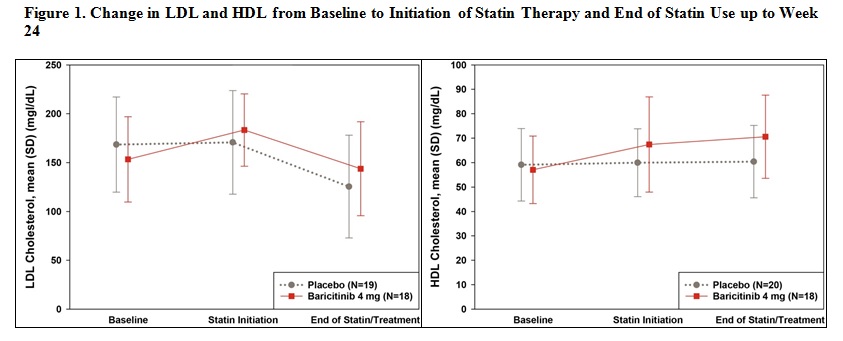
Results: Treatment with bari was associated with increased levels of total cholesterol, triglycerides (TG), low‑density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL‑C) with a stable LDL‑C/HDL‑C ratio. The lipid elevation plateaued after Wk 12 (Table 1). By NMR, bari did not significantly affect total LDL particle number while the small, dense potentially more atherogenic LDL particle numbers decreased. Bari significantly increased HDL-C, the total number of HDL particles, all HDL subfractions, and TG-rich lipoproteins. The magnitude of lipid change from baseline was not significantly different between baseline statin users and nonusers (Table 2). In bari patients who initiated statin therapy before Wk 24 (N=18), the increased levels of total cholesterol and LDL-C decreased to baseline levels by Wk 24, while HDL-C levels remained elevated after statin therapy (Figure 1). Similar results in lipids changes and response to initiation of statin therapy were observed for apolipoprotein A1 and apolipoprotein B corresponding to HDL-C and LDL-C.
Conclusion: These analyses showed that bari was associated with increased LDL-C, HDL-C, and TG levels, with a stable LDL-C/HDL-C ratio. The magnitude of lipid change was not significantly different between baseline statin users and nonusers. In patients who initiated statin therapy during the study, the elevation in total cholesterol, LDL-C, and TG decreased to pretreatment levels in response to statin therapy, while HDL-C remained elevated with statin therapy. References:
- McGrath et al. Curr Rheumatol Rep. 2015.
- Robertson et al. Nat Rev Rheumatol. 2013.
To cite this abstract in AMA style:
McInnes IB, Kremer J, Emery P, Zuckerman SH, Ruotolo G, Saifan C, Chen L, Thanabalasundrum S, Witt S, Macias W. Lipid Profile and Effect of Statin Treatment in Pooled Phase 2 and Phase 3 Baricitinib Studies [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/lipid-profile-and-effect-of-statin-treatment-in-pooled-phase-2-and-phase-3-baricitinib-studies/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lipid-profile-and-effect-of-statin-treatment-in-pooled-phase-2-and-phase-3-baricitinib-studies/