Session Information
Date: Tuesday, November 14, 2023
Title: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (AxSpA) have an increasing array of treatment options available, and new blood-based diagnostic tests to predict future treatment response are commercially available or are in development. Moreover, certain medications are restricted to patients who are already biologic experienced. We examined the point prevalence of the line of therapy (LoT) by type of arthritis to assess the size of the eligible patient population.
Methods: We queried the data warehouse of the Excellence Network in Rheumatology (ENRGY), a national practice-based research network (PBRN), to identify patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (AxSpA). Data from the electronic health record (EHR) was linked to a custom in-office tablet app that captures patients’ lifetime treatment history, disease duration and other disease features. We used both linked data sources to identify current users of conventional, biologic, or targeted synthetic DMARDS (cs/b/tsDMARDs) and classify them as to their prior treatment history over their lifetime. Results were reported by disease and by medication class
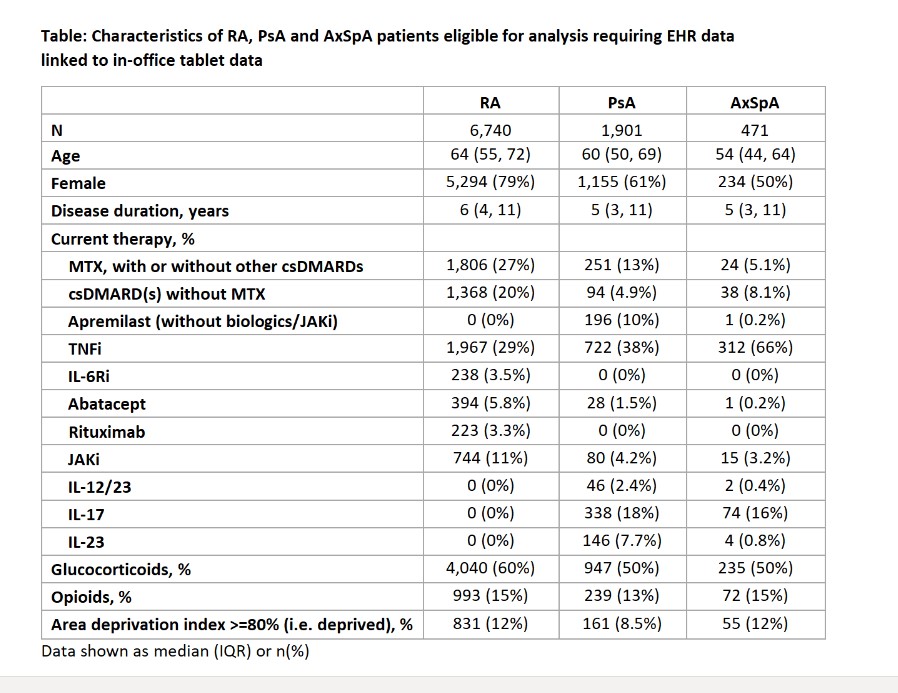
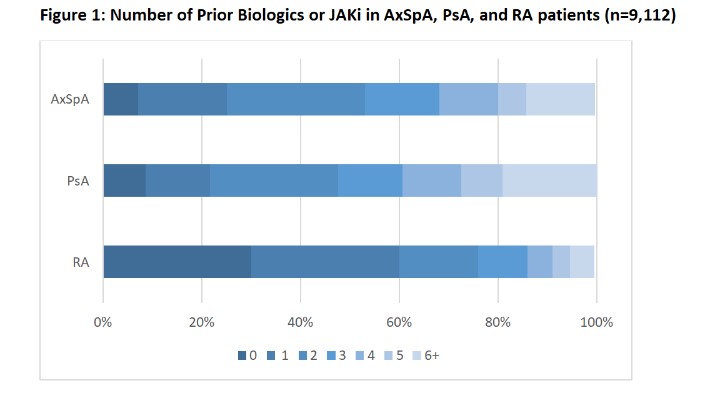
Results: As of Q4 2022, we identified 6,740 RA, 1,901 PsA, and 471 AxSpA patients who had linked EHR and self-reported lifetime treatment history data available who were current users of any cs/b/tsDMARDs (Table). The point prevalence of the number of prior biologics and JAKi is shown in the Figure. The proportion of patients who were biologic and JAKi naïve was 30% in RA, 9% in PsA, and 7% in AxSpA (Figure, left-most dark blue panel). The proportion of patients who were biologic naïve or treatment experienced with only 1 current or past biologic or JAKi was 25% in AxSpA, 22% in PsA, and 60% in RA.
Conclusion: Stakeholders interested in developing or marketing new therapies and diagnostic tests that target RA, PsA or AxSpA patients who are biologic naïve or minimally treatment experienced will be advantaged by these estimates as they estimate the potential opportunity and size of the eligible patient population. These proportions are being extrapolated to the U.S. to provide an estimate of the number of patients who might be eligible to receive various treatments or diagnostics that might be positioned or marketed according to line of therapy
To cite this abstract in AMA style:
Curtis J, Xie F, Su Y, Stewart P, Mudano A. Line of Therapy of Biologics and JAK Inhibitors in RA, PsA and AxSpA: Implications for Design and Uptake of New Drugs and Diagnostics [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/line-of-therapy-of-biologics-and-jak-inhibitors-in-ra-psa-and-axspa-implications-for-design-and-uptake-of-new-drugs-and-diagnostics/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/line-of-therapy-of-biologics-and-jak-inhibitors-in-ra-psa-and-axspa-implications-for-design-and-uptake-of-new-drugs-and-diagnostics/