Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Personalizing care in early psoriatic arthritis (PsA) is challenging. We set out to identify predictors of one-year response on the PASDAS using AI-based methods to guide treatment decisions and improve outcomes.
Methods: We analyzed the SPEED trial, a multicenter, open-label study of patients with early PsA and poor prognostic factors. Participants were randomized to standard step-up csDMARD therapy, combination csDMARD therapy, or early TNF inhibitor induction. Recursive partitioning (RPART) modeling identified predictors of PASDAS response at 48 weeks based on baseline variables, including BMI, treatment arm, baseline PASDAS, disease phenotype, disease duration, sex, age, education level, smoking status, and alcohol use. This approach mirrors clinical reasoning by breaking complex data into clear, logical steps, generating a decision tree based on real-world patient characteristics. The resulting tree begins with the most influential predictor and branches by relevant cutoffs. An importance plot showed each variable’s contribution to the outcome, and decision rules derived from the tree offer actionable insights to guide personalized treatment.
Results: Among 192 patients (median age 49 [IQR: 34–58]; median BMI 28.96 kg/m² [IQR: 26.06–33.63]), baseline PASDAS was 5.60 (IQR: 4.94–6.34), improving to 3.84 (IQR: 2.38–4.99) at week 48, with 37.1% achieving “good” PASDAS (< 3.2). Most (82.8%) had polyarticular involvement; 53.5% were non-smokers, and 64.7% reported alcohol use.BMI was deemed the most influential baseline predictor of lower PASDAS at 48 weeks (fig. 1); treatment ranked sixth. The RPART-derived prediction rules reveal important trends in treatment response (tbl. 1, fig. 2): (1) patients with BMI < 25 kg/m² and lower baseline disease activity (PASDAS < 5.4) experienced the most favorable outcomes (predicted PASDAS = 2.1) at week 48—regardless of treatment type; (2) among patients with higher baseline disease activity (PASDAS ≥ 5.4) and BMI < 27 kg/m², early TNF inhibitor therapy was associated with better outcomes (PASDAS = 2.6) compared to standard or combination csDMARD therapy (PASDAS = 4.0), highlighting the potential benefit of early biologic intervention; (3) poorer outcomes were observed in patients with higher BMI (≥ 27 kg/m²), longer disease duration (≥ 12 months), and polyarticular disease, particularly among those aged 51–74, with predicted PASDAS scores reaching as high as 6.0. Findings highlight the cumulative negative effect of obesity, chronicity, and disease severity.
Conclusion: BMI was the most influential predictor of achieving a lower PASDAS at one year, even more so than treatment exposure. As a modifiable factor, maintaining a BMI below 27 kg/m²—ideally under 25 kg/m²— can significantly improve patient outcomes. High baseline disease activity, obesity, and chronicity predicted poorer responses, and in these groups, treatment with early TNF inhibitors was superior to csDMARDs reinforcing the need for aggressive therapy in such patients. The predictive rules derived from this analysis offer clinicians valuable insights to support personalized care and encourage patients to prioritize weight management as a key component of their PsA treatment strategy.
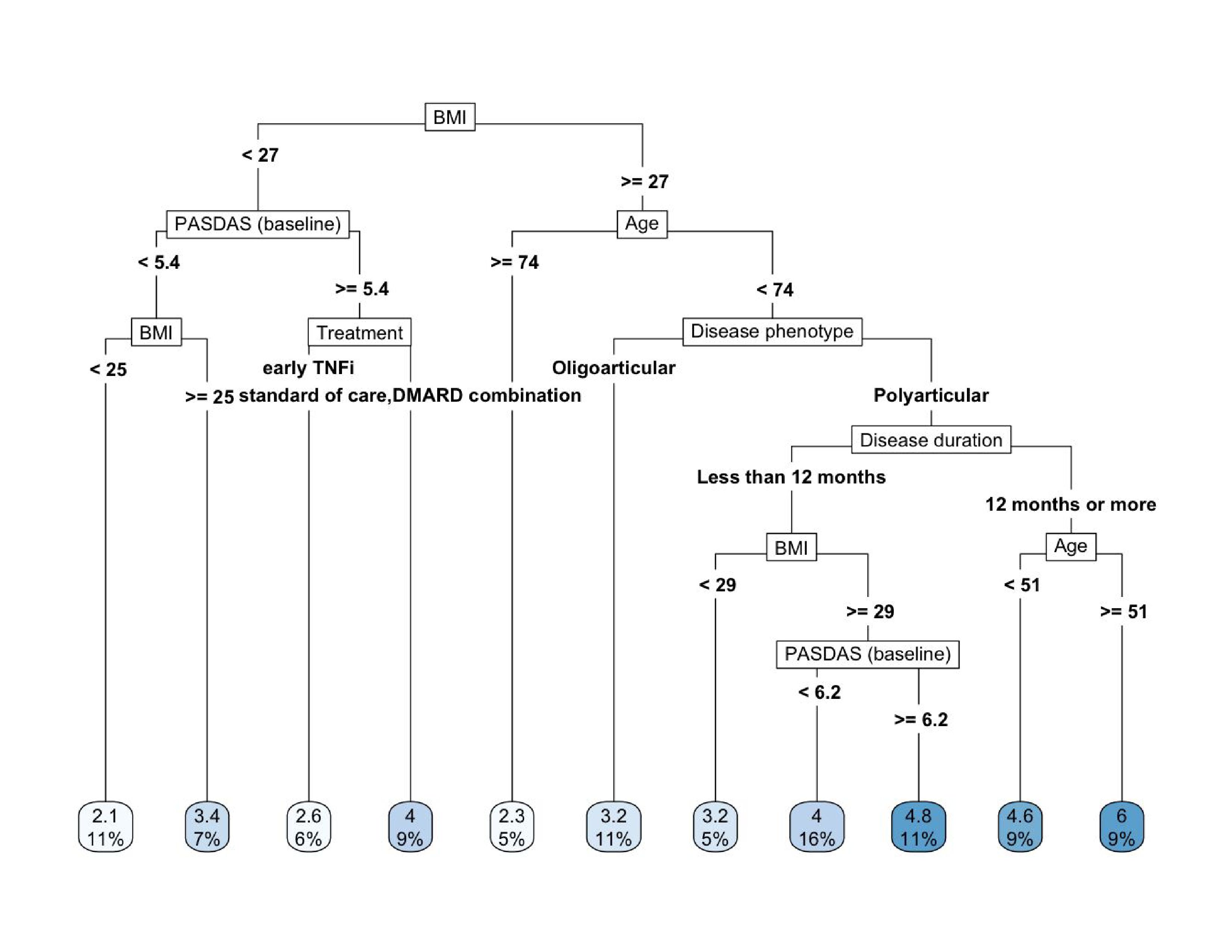 Table 1. Recursive partitioning-derived prediction rules for PASDAS at week 48
Table 1. Recursive partitioning-derived prediction rules for PASDAS at week 48
.jpg) Figure 1. Variable Importance Plot from Recursive Partitioning Model Identifying Key Predictors of ASDAS at One Year
Figure 1. Variable Importance Plot from Recursive Partitioning Model Identifying Key Predictors of ASDAS at One Year
.jpg) Figure 2. Decision Tree Plot Depicting Prediction Rules for One-Year PASDAS Based on BMI and Other Baseline Characteristics.
Figure 2. Decision Tree Plot Depicting Prediction Rules for One-Year PASDAS Based on BMI and Other Baseline Characteristics.
Each node represents a decision point based on key patient characteristics (e.g., BMI, baseline PASDAS). The final leaves show the predicted PASDAS score at 48 weeks, with the numbers representing the mean PASDAS value and the percentage indicating the proportion of patients in that group. To interpret the tree, follow the path from the root to the leaves, where each branch splits based on the patient’s characteristics, leading to a predicted treatment outcome.
To cite this abstract in AMA style:
Garaiman A, Massa S, Saeedi E, Gullick N, Tillett W, Hurtubise R, letarouilly j, Coates L. Lighten the Load: Artificial Intelligence Reveals Body Mass Index Outranks Treatment in One-Year Psoriatic Arthritis Outcomes—Findings from the SPEED Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/lighten-the-load-artificial-intelligence-reveals-body-mass-index-outranks-treatment-in-one-year-psoriatic-arthritis-outcomes-findings-from-the-speed-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lighten-the-load-artificial-intelligence-reveals-body-mass-index-outranks-treatment-in-one-year-psoriatic-arthritis-outcomes-findings-from-the-speed-trial/
