Session Information
Date: Monday, October 27, 2025
Title: Plenary II (0849–0854)
Session Type: Plenary Session
Session Time: 8:45AM-9:00AM
Background/Purpose: Bone marrow lesions (BMLs), detectable on MRI as areas of ill-defined high signal intensity on fluid-sensitive sequences, are a common feature of osteoarthritis (OA), representing areas of increased bone turnover, oedema, and fibrosis. BMLs are prevalent in ~80% of symptomatic knee OA patients, correlate with radiographic severity (Kellgren-Lawrence [KL] grade) and fluctuate with knee pain. LEVI-04, a first-in-class p75NTR-Fc fusion protein demonstrated significant and clinically meaningful improvements versus placebo for WOMAC pain, function and stiffness, patient global assessment (PGA) and pain on movement (StEPP).1 LEVI-04 was well tolerated, with no increased incidence of joint pathologies compared to placebo.1 This analysis investigated LEVI-04’s effects on BMLs and the relationship with OA symptoms.
Methods: 518 participants with symptomatic knee OA (WOMAC pain ≥ 4/10, KL grade ≥ 2) were enrolled in a Phase II multicentre randomized double-blinded placebo-controlled trial. Participants received placebo or LEVI-04 (0.3, 1, or 2 mg/kg) every 4 weeks through week 16. BML area (mm²) was measured in a blinded fashion from coronal proton density-weighted fat-suppressed (PD-FS) sequences (slice thickness 3 mm, TE/TR 35/3000 ms) of the target knee at baseline and week 20. For each participant, BML area was determined as the largest area within the MRI sequence of ill-defined high signal intensity of the subchondral bone marrow, and without presence of a fracture line. The perimeter of each BML was highlighted and the area measured electronically using IAG Dynamika™ Software. For BML presence, participants were categorized as BML positive if ≥1 lesion was identified in the target knee. Presence and change from baseline in BML area were assessed in response to LEVI-04. Spearman’s Rho correlation was used to assess the relationship between changes in BML area and changes in clinical outcomes.
Results: Presence of BMLs at baseline was similar across LEVI-04 and placebo groups (74-79%). At week 20, there was a significant, dose-dependent reduction in the proportion of participants with BMLs (figure 1) and in mean BML area from baseline (0.3 mg/kg p< 0.01; 1 mg/kg and 2 mg/kg p< 0.001) compared to placebo. A greater absolute reduction in BML area was seen in patients with higher baseline KL grades (figure 2). Comparison between change from baseline in BML area and clinical symptoms for all LEVI-04 groups showed modest yet statistically significant positive correlations (p< 0.001) for WOMAC pain (Rho=0.21), function (Rho=0.22) and stiffness (Rho=0.19), PGA (Rho=0.20), and StEPP (Rho=0.25) (figure 3).
Conclusion: A significant, dose-dependent reduction in BML presence and area across all KL grades was observed for LEVI-04 compared with placebo. Changes in BML area significantly correlated with changes in patient-reported symptoms. LEVI-04 supplements endogenous soluble p75NTR, which is present in the extracellular matrix of OA tissue including BMLs. These findings suggest that LEVI-04 holds promise as a therapy to provide contemporaneous modification of structure (BMLs) and symptoms of OA.< !Reference: 1. Conaghan P, et al. Arthritis Rheumatol. 2024;76 (suppl 9)
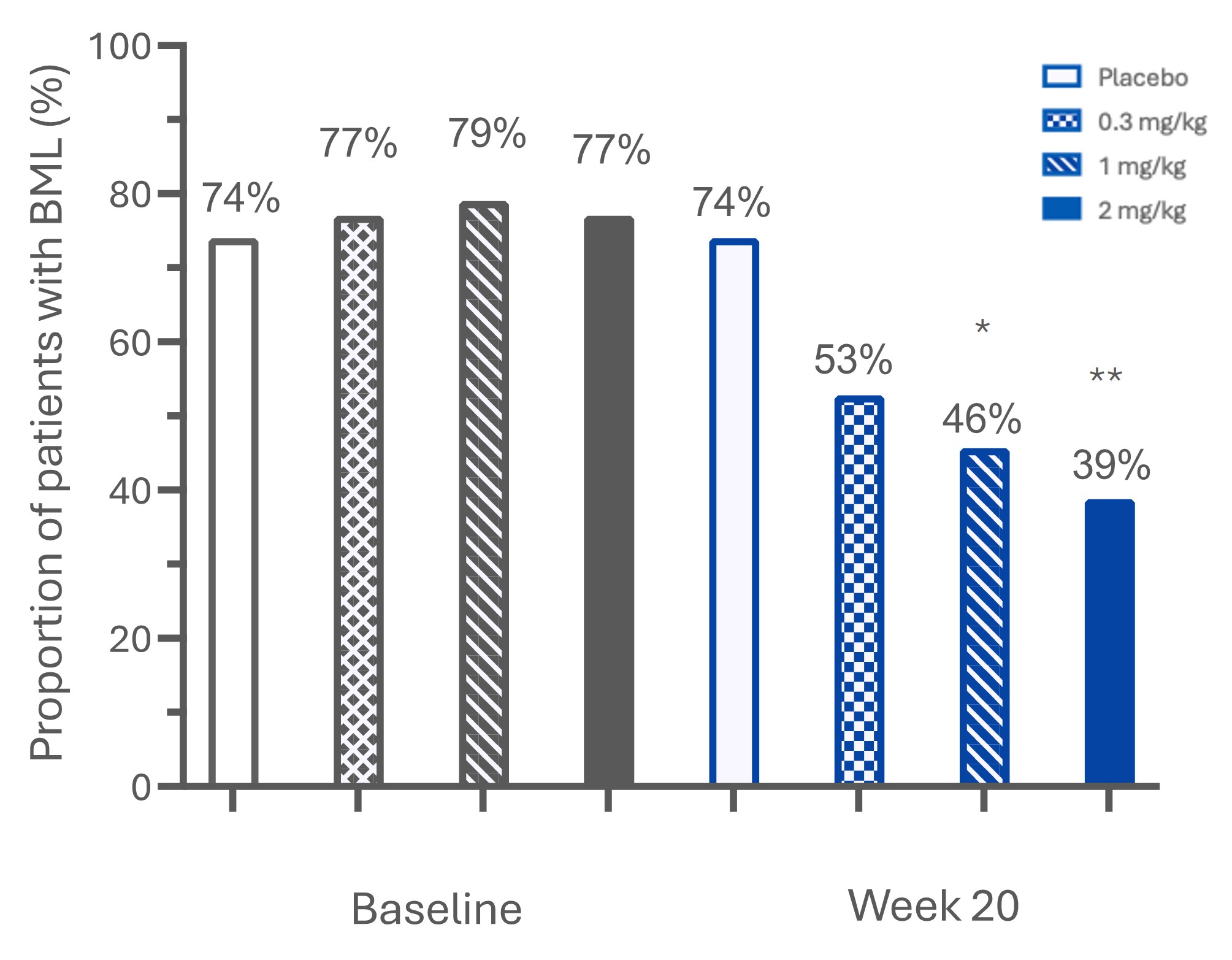 Proportion of participants with bone marrow lesions at baseline and week 20. *P < 0.05; **P < 0.01 vs placebo at 20 weeks
Proportion of participants with bone marrow lesions at baseline and week 20. *P < 0.05; **P < 0.01 vs placebo at 20 weeks
.jpg) Change from baseline in bone marrow lesion area (mm2) in placebo or LEVI-04 0.3, 1 or 2 mg/kg groups at week 20. *P < 0.05, **P < 0.01; ***P < 0.001 vs placebo
Change from baseline in bone marrow lesion area (mm2) in placebo or LEVI-04 0.3, 1 or 2 mg/kg groups at week 20. *P < 0.05, **P < 0.01; ***P < 0.001 vs placebo
.jpg) FIGURE 3: Spearman’s Rho correlations between change from baseline in BML area and OA symptoms.
FIGURE 3: Spearman’s Rho correlations between change from baseline in BML area and OA symptoms.
To cite this abstract in AMA style:
Westbrook S, Guermazi A, Conaghan P. LEVI-04 Significantly Reduces Bone Marrow Lesions and Symptoms in Knee Osteoarthritis: Results from a Phase II RCT [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/levi-04-significantly-reduces-bone-marrow-lesions-and-symptoms-in-knee-osteoarthritis-results-from-a-phase-ii-rct/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/levi-04-significantly-reduces-bone-marrow-lesions-and-symptoms-in-knee-osteoarthritis-results-from-a-phase-ii-rct/
