Session Information
Session Type: Late-Breaking Abstracts
Session Time: 8:00AM-9:30AM
Background/Purpose: There is an urgent need for new therapies to treat OA. Excess neurotrophins (NT) are implicated in OA and other painful conditions. Previous OA therapies selectively targeting NGF provided analgesia but were associated with significant joint pathologies. LEVI-04, a p75NTR-Fc, first-in-class fusion protein, supplements endogenous p75NTR, modulating neurotrophin levels, and inhibiting NT3. Preclinical studies supported analgesic efficacy and Phase I trials demonstrated safety and linear pharmacokinetics. This phase II RCT aimed to assess efficacy and safety in people with knee OA.
Methods: This was a 20-week PhII multicentre (Europe and Hong Kong) RCT in people with painful (≥4/10 WOMAC), radiographic (KL≥2) knee OA. Participants were randomised to baseline then 4-weekly IV placebo or 0.3, 0.1 or 2 mg/kg LEVI-04 through wk16, with safety follow-up to wk30. The primary endpoint was change in WOMAC pain to wk17, with secondary outcomes including function, Patient Global Assessment and >50% pain responders. X-rays of 6 large joints and MRI of knees were utilised for inclusion/exclusion criteria at baseline, and safety evaluation at wk20. ITT analyses were performed using an ANCOVA model (covariates were baseline WOMAC score and site), with Dunnett’s step-down testing procedure comparing the 3 active arms versus placebo.
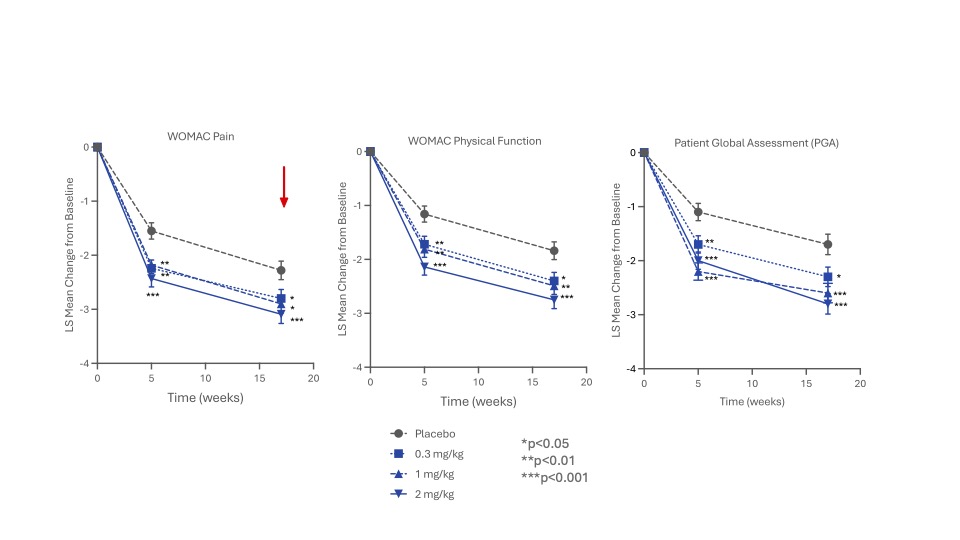
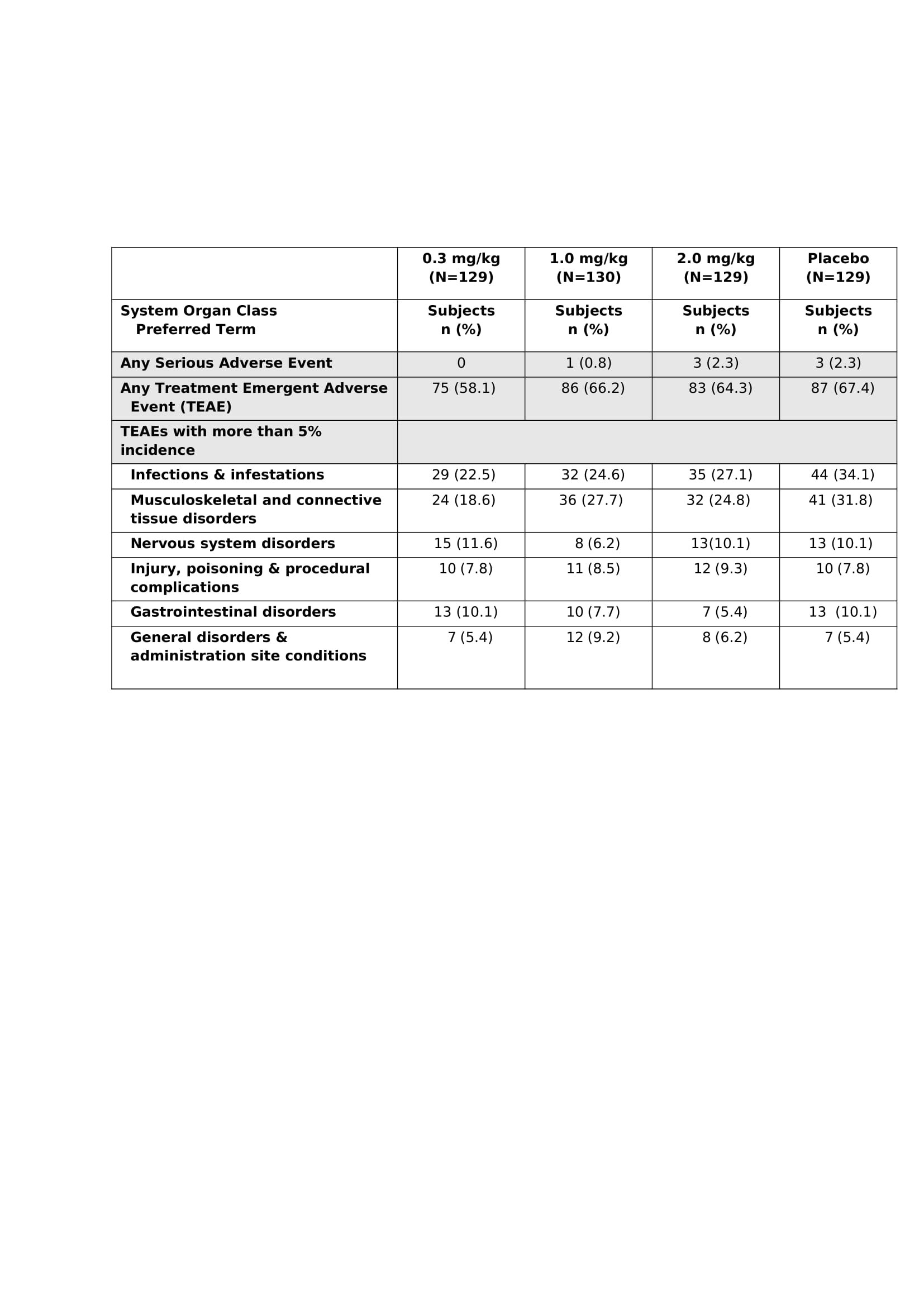
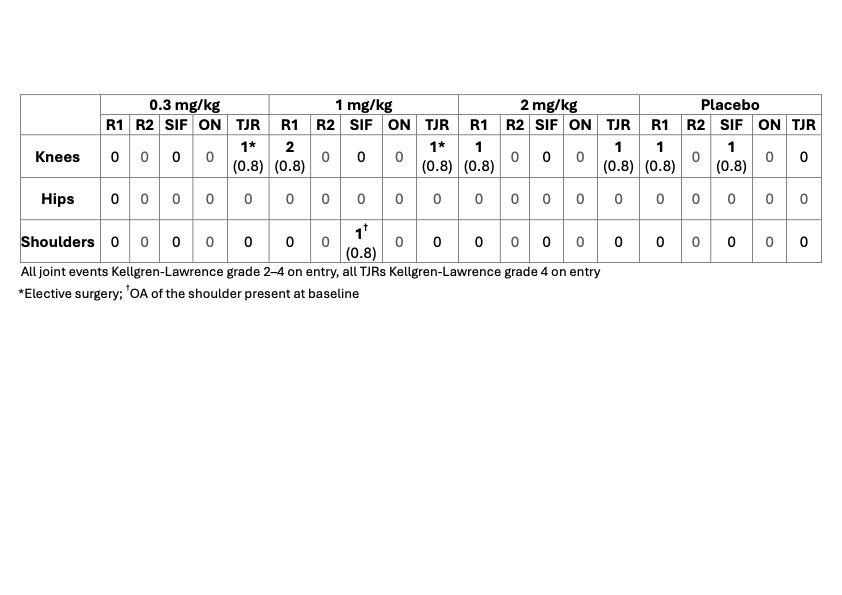
Results: 518 people with knee OA were enrolled, balanced across groups (mean age 63.1–65.4 years, mean BMI 29.3–30.3, female participants 51.5–61.5%). Statistical significance was met for the primary and secondary efficacy endpoints (Figure 1) at wk5 and wk17 (p< 0.05 vs placebo, all doses). More than 50% of the LEVI-04-treated patients reported ≥50% reduction in pain and >25% reported ≥75% reduction at weeks 5 and 17. LEVI-04 was well tolerated, with no increased incidence of SAEs, TEAEs and joint pathologies including rapidly progressive OA (Tables 2 and 3) compared to placebo. Incidence of anti-drug antibodies was low: 9 participants tested positive pre-dosing, 6 at week 5 or week 20, all at the lowest limit of detection.
Conclusion: LEVI-04 demonstrated significant and clinically meaningful improvement in pain, function and other outcomes. LEVI-04 was well tolerated at all doses studied, supporting the concept of supplementing endogenous p75NTR as a treatment for OA and other pain conditions. Phase III trials are in planning.
To cite this abstract in AMA style:
Conaghan P, Guermazi A, Katz N, Bihlet A, Rom D, Perkins M, Hughes B, Herholdt C, Bombelka I, Westbrook S. LEVI-04, a Novel neurotrophin-3 Inhibitor, Substantially Improves Pain and Function Without Deleterious Effects on Joint Structure in People with Knee Osteoarthritis: A Randomized Controlled Phase II Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/levi-04-a-novel-neurotrophin-3-inhibitor-substantially-improves-pain-and-function-without-deleterious-effects-on-joint-structure-in-people-with-knee-osteoarthritis-a-randomized-controlled-phase-ii/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/levi-04-a-novel-neurotrophin-3-inhibitor-substantially-improves-pain-and-function-without-deleterious-effects-on-joint-structure-in-people-with-knee-osteoarthritis-a-randomized-controlled-phase-ii/