Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
– Background/Purpose: Tumor necrosis factor (TNF) alpha, a key proinflammatory cytokine in rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), has been a major target in the treatment of these conditions. Although the immunosuppressant side effects are well-known, recent studies suggest that TNF alpha inhibition is directly linked to the development of leukopenia and that serial complete blood cell (CBC) counts should be monitored. However, most studies investigating hematologic side effects of anti-TNF alpha therapy have been case studies. This study was designed to identify the frequency of leukopenia in patients on anti-TNF alpha therapy at a tertiary care institution and whether certain demographics, baseline white blood cell (WBC) counts, or other factors correlate with the development of leukopenia.
– Methods: The chart review was performed for adult patients who received anti-TNF alpha therapy (adalimumab, etanercept, certolizumab, golimumab, or infliximab) at Loyola University Medical Center between 2007 and 2016 and who had a baseline WBC count in the year prior to therapy. Subjects with baseline leukopenia (WBC <4 K/uL) were excluded. Data analysis was performed using the chi-square test, analysis of variance, Student t-test, and a multivariable general linear model.
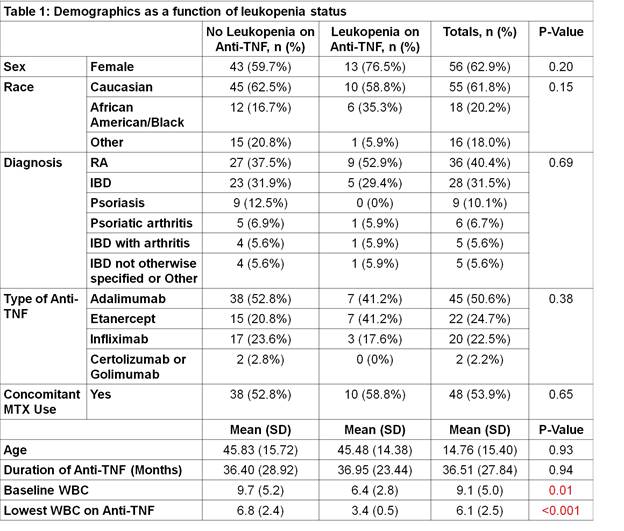
– Results: Of 89 patients who met the study criteria, 17 patients (19%) developed leukopenia during anti-TNF alpha therapy. Patients in the study were treated for RA, IBD, psoriasis, psoriatic arthritis, IBD with associated arthritis, inflammatory arthritis not otherwise specified, or other diagnoses. Patients who developed leukopenia had significantly lower mean WBC counts compared to those who did not develop leukopenia (p = 0.01) while all other factors including sex, race, age, type of TNF inhibitor, diagnosis, duration of therapy, and concomitant methotrexate use were comparable between the two groups (Table 1). After controlling for these variables, baseline WBC count was the only significant predictor of lowest WBC count. For every one unit (K/uL) decrease in baseline WBC, the lowest WBC on therapy decreased by 0.35 K/uL (p<0.001).
– Conclusion: In summary, leukopenia developed in a considerable proportion of patients on anti-TNF alpha therapy. This observation was not associated with several variables studied, including type of TNF inhibitor used, diagnosis, duration of therapy, and concomitant use of methotrexate. A lower baseline WBC count was the only significant predictor for both the development of leukopenia and of the greatest degree of leukopenia. These findings suggest that leukopenia should be screened periodically for all patients on TNF alpha inhibitor therapy regardless of baseline demographics and that those who have lower baseline WBC counts may warrant closer monitoring.
To cite this abstract in AMA style:
Xiong W, Ostrowski RA, Adams W, Tehrani R. Leukopenia and Tumor Necrosis Factor Alpha Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/leukopenia-and-tumor-necrosis-factor-alpha-inhibitor-therapy/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/leukopenia-and-tumor-necrosis-factor-alpha-inhibitor-therapy/