Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The market introduction of biosimilars has generated the need for novel biologic naming conventions, in part to support pharmacovigilance. We evaluated the familiarity of health care practitioners and other clinical professionals with biosimilars and their perceptions regarding the Food and Drug Administration’s (FDA’s) nomenclature for biologics in clinical practice. We hypothesized that health care providers would be unfamiliar with key concepts surrounding biosimilars and the FDA four-character suffix and would vary in their perceived value of the naming guidance in clinical practice.
Methods: A survey was emailed every two weeks over a two-month period to health care professionals within two large health care systems in the Philadelphia area: University of Pennsylvania Health System and the Corporal Michael J. Crescenz VA Medical Center. The survey was sent to prescribers and other health care administrators such as pharmacists, nurses, and medical coders. Prescribers received a survey if they had previously prescribed at least one biosimilar therapy from a query of prescription data from the electronic medical record. The survey assessed knowledge of key aspects of biosimilar therapies and the perceived utility of the FDA naming convention in practice. Differences in responses across prespecified sub-groups were tested using c2 and Fisher’s exact tests.
Results: The survey was sent to 506 prescribers and other clinical staff; 50 were returned from inactive email addresses. Of the remaining 456, 83 (~18%) responded. Most were from the University of Pennsylvania, with the highest number of responses coming from the Divisions of Hematology/Oncology and Rheumatology, and the Department of Pharmacy. Of respondents that identified being ‘out of training’, 14 (45%) had >10 years of clinical experience. Of those still ‘in training’, 27 (93%) were residents or fellows. Although respondents had a general understanding of the regulatory criteria for approval, knowledge about biosimilars was generally poor (Table 1). For example, only 52% of all respondents correctly identified that a biosimilar differed from a generic drug. When asked about biosimilar naming conventions, 67% reported using the brand name in clinical practice to distinguish between biosimilars and reference products. In contrast, as shown in Figure 1 (Q1), only a minority of respondents reported regular use of the four-character suffix to identify biologic therapies. Overall, there was little agreement on the utility of the four-character suffix in many aspects of care. For example, a majority of respondents (68% overall) indicated a neutral response when asked if the four-character suffix promoted medical errors (Figure 2, N5). Most prescribers (76%) disagreed that the 4-character suffix affected their decision to use biosimilars.
Conclusion: These survey findings suggest a knowledge gap with regard to biosimilars and lack of consensus about the usefulness of employing the four-character suffix in clinical practice. The lack of overall familiarity with biosimilars may also have contributed to a lack of comfort with the naming convention.
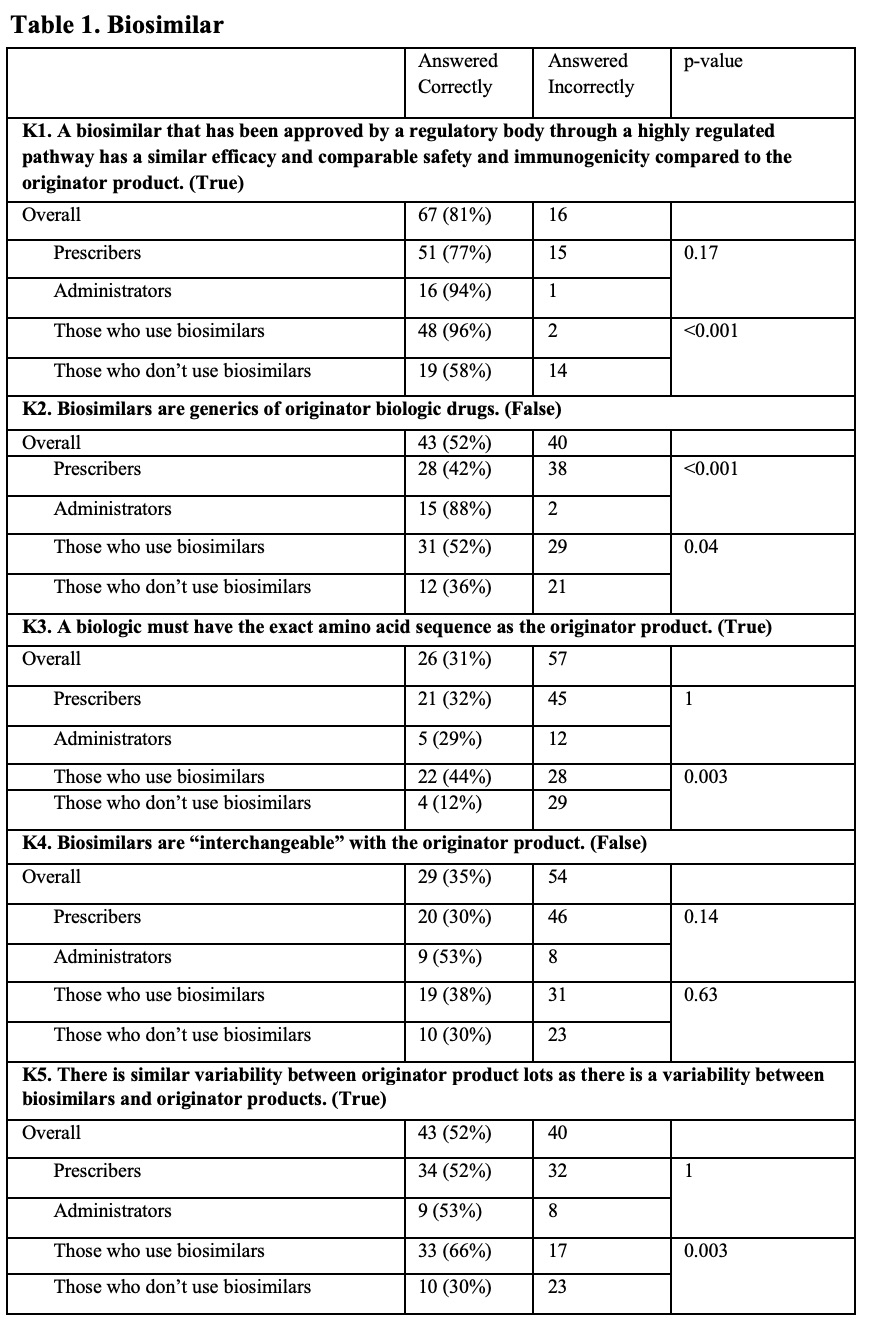 This table includes the results of True and False questions designed to test knowledge of basic concepts of biosimilar therapies. All respondents are stratified by Prescribers vs. Administrators, and Those who use biosimilars vs. Those who don’t use biosimilars in practice. For the purposes of these questions, “Those who use biosimilar drugs” includes both Prescribers as well as Administrators who encounter biologics. The correct answer is included at the end of the question. Total correct answers shown alongside in percentages. Incorrect totals include any marked ‘Unsure’ responses.
This table includes the results of True and False questions designed to test knowledge of basic concepts of biosimilar therapies. All respondents are stratified by Prescribers vs. Administrators, and Those who use biosimilars vs. Those who don’t use biosimilars in practice. For the purposes of these questions, “Those who use biosimilar drugs” includes both Prescribers as well as Administrators who encounter biologics. The correct answer is included at the end of the question. Total correct answers shown alongside in percentages. Incorrect totals include any marked ‘Unsure’ responses.
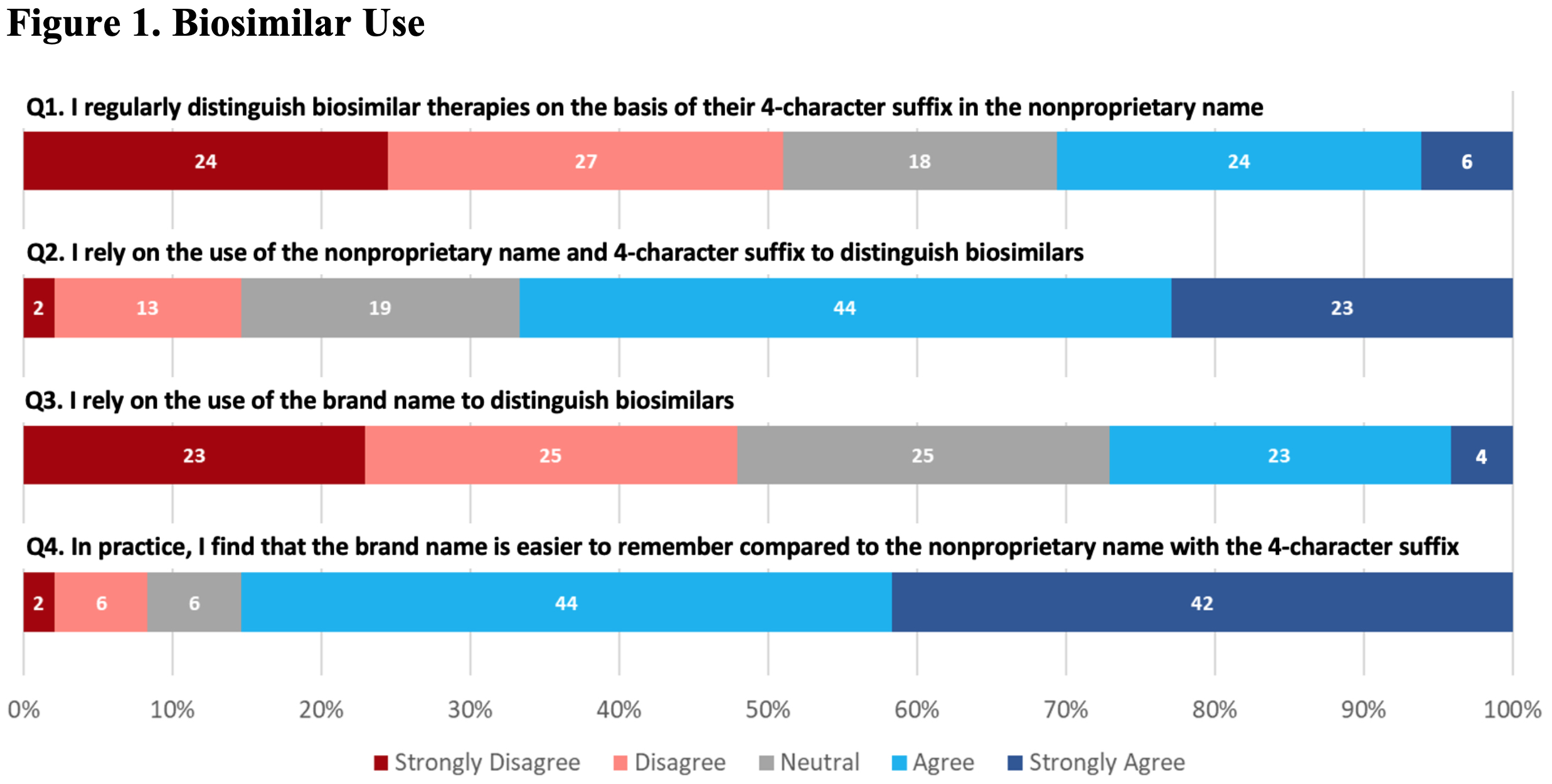 Questions related to general use of biosimilar identifiers in clinical practice. All questions included: To what degree do you agree with the following?
Questions related to general use of biosimilar identifiers in clinical practice. All questions included: To what degree do you agree with the following?
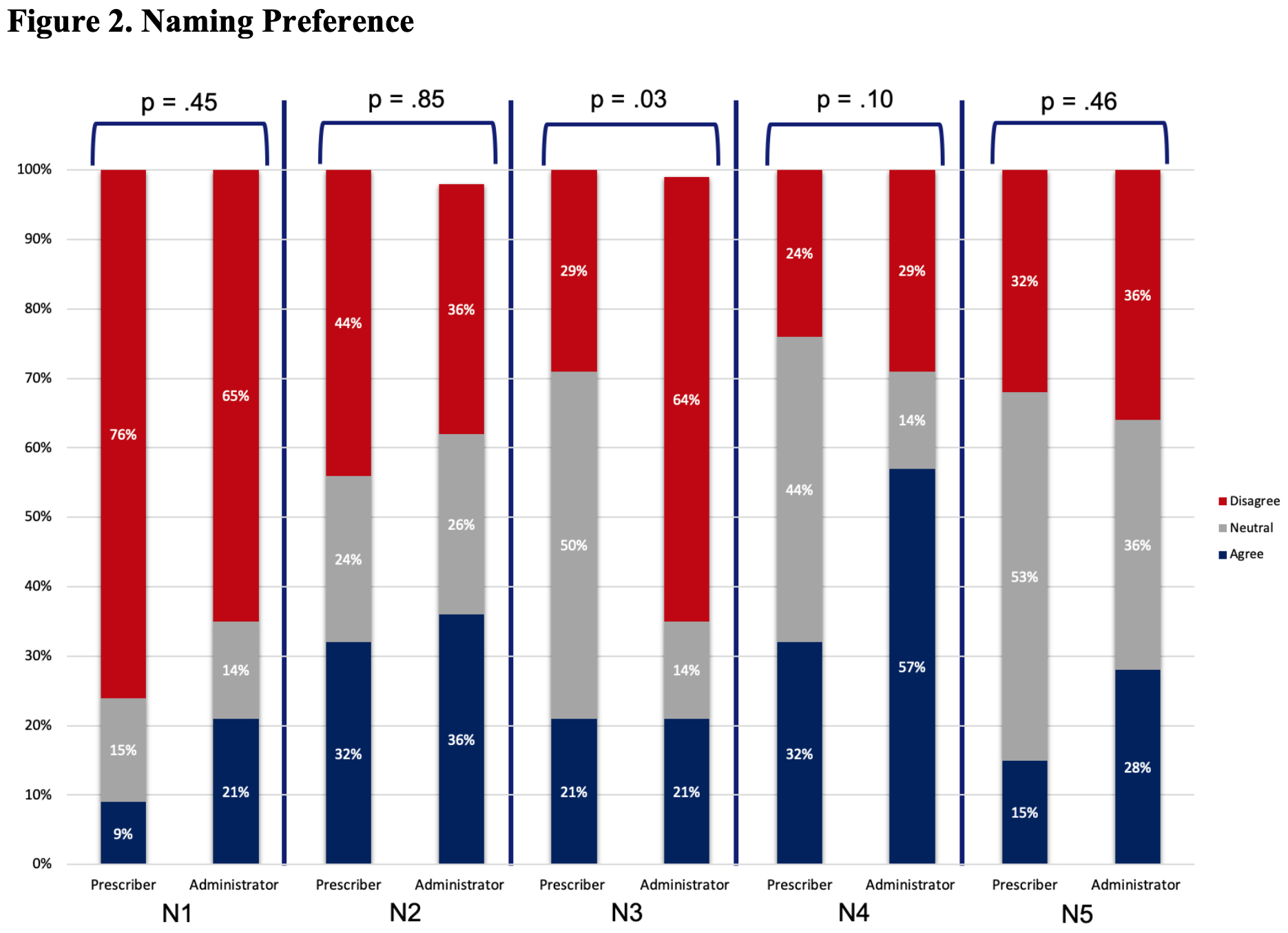 Questions regarding naming preferences. All questions included: To what degree do you agree with the following? The questions are detailed below: N1. I am less likely to use biosimilars compared to the originator product because the nonproprietary names of biosimilars are different from the originator product. N2. I have experienced that the use of brand names to distinguish biosimilars introduces commercial bias in clinical practice. N3. The 4-character suffix in the non-proprietary name influences patients to think there are important differences between biosimilars and the originator products. N4. The 4-character suffix in the nonproprietary name causes inefficiencies due to inconsistencies with other naming systems in practice. N5. The use of the 4-character suffix in the nonproprietary name to distinguish biosimilar therapies in practice promotes medication errors in the ordering of biologics.
Questions regarding naming preferences. All questions included: To what degree do you agree with the following? The questions are detailed below: N1. I am less likely to use biosimilars compared to the originator product because the nonproprietary names of biosimilars are different from the originator product. N2. I have experienced that the use of brand names to distinguish biosimilars introduces commercial bias in clinical practice. N3. The 4-character suffix in the non-proprietary name influences patients to think there are important differences between biosimilars and the originator products. N4. The 4-character suffix in the nonproprietary name causes inefficiencies due to inconsistencies with other naming systems in practice. N5. The use of the 4-character suffix in the nonproprietary name to distinguish biosimilar therapies in practice promotes medication errors in the ordering of biologics.
To cite this abstract in AMA style:
Lavery C, Olave M, Leonard C, Lo Re V, Kay J, Baker J. Knowledge of Biosimilars and Perceptions of Biosimilar Naming Conventions in Clinical Practice [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/knowledge-of-biosimilars-and-perceptions-of-biosimilar-naming-conventions-in-clinical-practice/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/knowledge-of-biosimilars-and-perceptions-of-biosimilar-naming-conventions-in-clinical-practice/
