Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
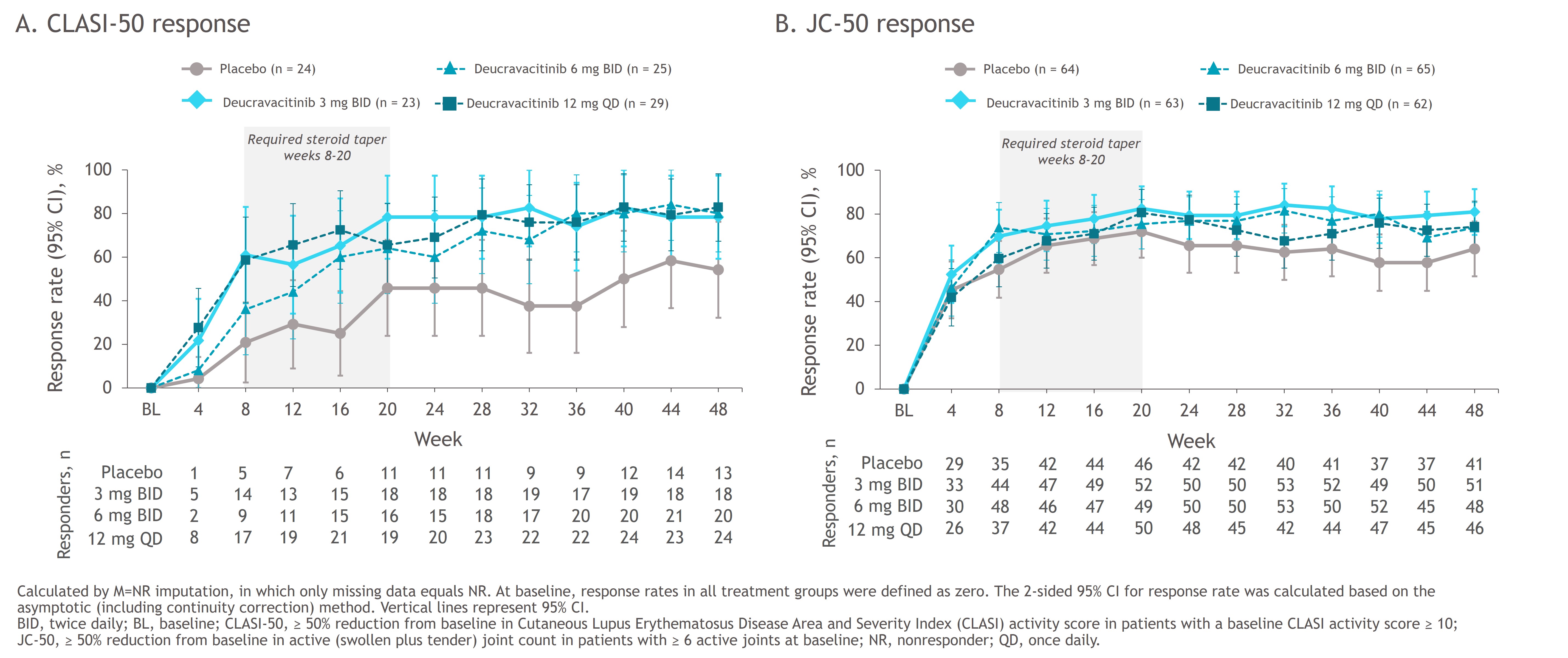
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor approved in multiple countries for the treatment of moderate to severe plaque psoriasis. The 48-week, double-blind, phase 2 PAISLEY trial in patients with active SLE (NCT03252587) met its primary endpoint and all key secondary endpoints at the deucravacitinib 3-mg twice-daily (BID) dose vs placebo. In this post hoc analysis, response rates over time were evaluated for ≥ 50% reduction from baseline in Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) activity score in patients with a baseline CLASI activity score ≥ 10 (CLASI-50), ≥ 50% reduction from baseline in active (swollen plus tender) joint count in patients with ≥ 6 active joints at baseline (JC-50), and other key secondary endpoints.
Methods: Patients with active SLE receiving standard of care were randomized 1:1:1:1 to placebo (n = 90) or deucravacitinib 3 mg BID (n = 91), 6 mg BID (n = 93), or 12 mg once daily (QD; n = 89). Previous reporting from PAISLEY used strict nonresponder imputation criteria with several conditions considered a nonresponse applied after week 20, including not achieving a glucocorticoid dose of ≤ 7.5 mg/day by week 20, resulting in different imputation methods used before vs after week 20. Here, we characterize disease activity improvement in 2 key lupus manifestations, imputing only missing data as nonresponse (M=NR) through week 48 and assessing response rate kinetics using the same imputation method throughout.
Results: Patients receiving deucravacitinib had numerically higher CLASI-50 response rates than those receiving placebo starting at week 4 (placebo, 4.2%; 3 mg BID, 21.7%; 6 mg BID, 8.0%; 12 mg QD, 27.6%) (Figure 1A); robust differences were maintained through week 48. Numerical differences in JC-50 response rates were first observed at week 8 (placebo, 54.7%; 3 mg BID, 69.8%; 6 mg BID, 73.8%; 12 mg QD, 59.7%) and became more notable from week 24 (Figure 1B). Differences in responses of both organs were maintained with deucravacitinib beyond week 20, after the protocol-mandated glucocorticoid taper.
Conclusion: These data suggest that higher response rates with deucravacitinib vs placebo occur early on for mucocutaneous and somewhat later for musculoskeletal manifestations. Permitted concomitant glucocorticoid use may partially explain the placebo group responses. Robust differences were seen after the glucocorticoid taper, further supporting the efficacy of deucravacitinib.
To cite this abstract in AMA style:
Van Vollenhoven R, Merola J, Dao K, Leszczynski P, Pike M, Pomponi S, Hobar C, Colombo M, Koti R, Banerjee S, Wegman T, Morand E. Kinetics of Mucocutaneous and Musculoskeletal Responses to Deucravacitinibin Patients with Active SLE in the Phase 2 PAISLEY Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/kinetics-of-mucocutaneous-and-musculoskeletal-responses-to-deucravacitinibin-patients-with-active-sle-in-the-phase-2-paisley-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/kinetics-of-mucocutaneous-and-musculoskeletal-responses-to-deucravacitinibin-patients-with-active-sle-in-the-phase-2-paisley-trial/