Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Preservation of long-term kidney function and sustained reduction of glucocorticoid use are major therapeutic goals in lupus nephritis (LN). In the randomized, double-blind, placebo-controlled, Phase 2 NOBILITY trial (NCT02550652), patients with proliferative LN receiving obinutuzumab with standard-of-care therapy showed clinically meaningful improvement in complete and overall renal responses (CRR and ORR) at Weeks 52, 76 and 104 compared with those receiving placebo and standard-of-care therapy.1 We conducted a post hoc analysis of NOBILITY to assess kidney-related outcomes and steroid-sparing effects.
Methods: Cox regression analysis was conducted for the time to first kidney-related event (death, doubling of serum creatinine and treatment failure), LN flare and first 30% and 40% eGFR decline from baseline. The eGFR slope was assessed in a linear mixed-effects model. Assessment of a steroid-sparing effect of ≤7.5 mg/day of prednisone while achieving CRR at Weeks 76 and 104 without use of >7.5 mg/day of prednisone between Weeks 64 and 76 and Weeks 92 and 104, respectively, was calculated by the Cochran–Mantel–Haenszel test stratified for race (Afro-Caribbean/African American vs others) and country (US vs non-US). Glucocorticoid dose was imputed by the LOCF method for patients who discontinued the study and/or for missing data. All patients met ACR classification criteria for systemic lupus erythematosus and had biopsy-proven proliferative LN.
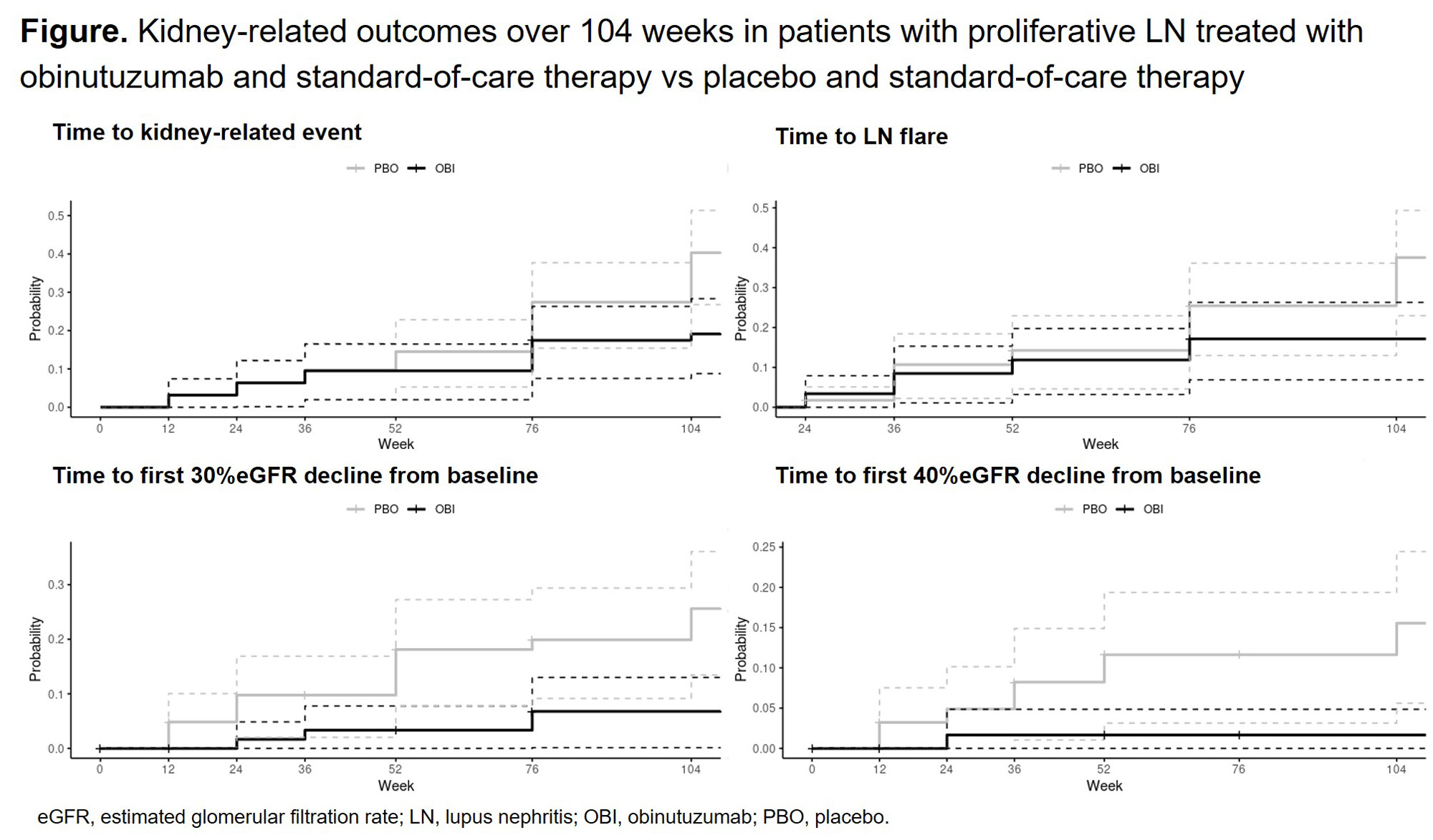
Results: Obinutuzumab significantly reduced the risk of kidney-related events or death (HR, 0.40; 95% CI, 0.20 to 0.80), LN flare (HR, 0.43; 95% CI, 0.20 to 0.95) and time to first eGFR decline of 30% (HR, 0.20; 95% CI, 0.06 to 0.61) and 40% (HR, 0.09; 95% CI, 0.01 to 0.73) (Figure). Risk of sustained eGFR decline of 30% and 40% was numerically lower, and a significant difference in attenuation of eGFR slope decline was observed between patients receiving obinutuzumab and standard-of-care therapy and those receiving placebo and standard-of-care therapy (annual slope difference, 4.10 mL/min/year; 95% CI, 0.14 to 8.08). Patients receiving obinutuzumab and standard-of-care therapy were significantly more likely to achieve CRR at Week 76 without receiving >7.5 mg/day of prednisone from Week 64 through 76 than those receiving placebo and standard-of-care therapy (24 of 63 patients [38.1%] vs 10 of 62 [16.1%], respectively; P< 0.01). A similar trend was observed at Week 104 without receiving >7.5 mg/day of prednisone from Week 92 through 104, but this did not reach statistical significance.
Conclusion: Obinutuzumab, in addition to increasing the possibility of achieving CRR, significantly reduced the risk of kidney-related events, eGFR decline, time to LN flare and eGFR slope decline in a post hoc analysis, suggesting that obinutuzumab in combination with standard-of-care therapy may positively impact kidney-related outcomes. In addition, a significant steroid-sparing effect was observed at Week 76. Obinutuzumab is being evaluated in patients with active proliferative LN in the global registrational Phase 3 REGENCY trial (NCT04221477).
Reference
1. Furie RA, et al. Ann Rheum Dis. 2022;81:100-107.
To cite this abstract in AMA style:
Rovin B, Ross Terres J, Giang S, Schindler T, Turchetta A, Garg J, Furie R, Pendergraft III W, Malvar A. Kidney-Related Outcomes and Steroid-Sparing Effects in Patients with Active Lupus Nephritis Treated with Obinutuzumab: A Post Hoc Analysis of a Phase 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/kidney-related-outcomes-and-steroid-sparing-effects-in-patients-with-active-lupus-nephritis-treated-with-obinutuzumab-a-post-hoc-analysis-of-a-phase-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/kidney-related-outcomes-and-steroid-sparing-effects-in-patients-with-active-lupus-nephritis-treated-with-obinutuzumab-a-post-hoc-analysis-of-a-phase-2-trial/