Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Baricitinib, a selective JAK1/JAK2 inhibitor, has demonstrated efficacy in rheumatoid arthritis (RA). In patients with stable disease control for at least six months, reducing the dose by half (dosage optimization, OP) may improve safety and cost-effectiveness. Since the literature on this approach is limited, it is crucial to analyze the factors that influence the time required to implement this optimization.
Methods: A single-center retrospective study was conducted at the Donostia University Hospital, Spain, in patients with rheumatoid arthritis (RA) treated with Baricitinib between October 2017 and December 2023. Demographic and clinical-analytical variables were analyzed to identify factors associated with dose optimization. Using logistic regression and survival analysis with Cox regression, the probability and time to dose reduction were evaluated. Furthermore, safety and tolerance were compared between patients with optimized dose (OP) and full dose (DP), providing information on the clinical benefits of therapeutic optimization.
Results: 149 patients were found, mostly women (79.9%), with a mean age of 63.3 years, 38 were optimized (OP), while 111 continued with full dose (DP). The duration of treatment was significantly longer in the OP group, with a mean of 4.6 years compared to 2.6 years in the DP group (p = 0.0001) (Table 1).In the univariate and multivariate analysis using logistic regression, no variable showed a statistically significant influence on the probability of dose optimization. However, in Cox regression analysis, the presence of anti-CCP antibodies was associated with a higher likelihood of optimization (HR = 2.12; 95% CI: 1.08 – 4.15; p = 0.028) (Table 2).No significant difference in safety was observed between the two groups, indicating comparable outcomes (Table 3).
Conclusion: Baricitinib dose optimization appears to be influenced by the presence of anti-CCP antibodies, while factors such as previous treatments, number of drugs used, monotherapy and markers of poor prognosis did not show a significant association. Furthermore, patients on optimized doses demonstrated better tolerance to the treatment without an increase in adverse effects supporting its safety and clinical benefit in the management of rheumatoid arthritis.
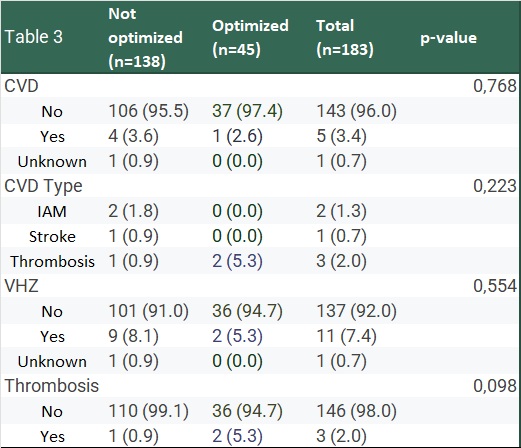 Table 1. Baseline characteristics, comorbidities, treatment history, and clinical outcomes of patients receiving Baricitinib therapy, categorized by treatment optimization status (Not optimized vs. Optimized).
Table 1. Baseline characteristics, comorbidities, treatment history, and clinical outcomes of patients receiving Baricitinib therapy, categorized by treatment optimization status (Not optimized vs. Optimized).
.jpg) Table 2. Multivariate Cox regression analysis of factors associated with treatment outcomes in patients receiving Baricitinib, including demographic variables, disease duration, serological markers, smoking status, and prior targeted therapies. Anti-CCP antibody (CCP). Disease-modifying antirheumatic drugs (DMARDs)
Table 2. Multivariate Cox regression analysis of factors associated with treatment outcomes in patients receiving Baricitinib, including demographic variables, disease duration, serological markers, smoking status, and prior targeted therapies. Anti-CCP antibody (CCP). Disease-modifying antirheumatic drugs (DMARDs)
.jpg) Table 3. Cardiovascular disease (CVD), CVD type, herpes zoster (VHZ), and thrombosis events in patients treated with Baricitinib, comparing optimized and not optimized treatment groups.
Table 3. Cardiovascular disease (CVD), CVD type, herpes zoster (VHZ), and thrombosis events in patients treated with Baricitinib, comparing optimized and not optimized treatment groups.
To cite this abstract in AMA style:
Campos-Martin D, Alcorta-Lorenzo N, Egües Dubuc C, Lopez-Dominguez L, Otero L, Sánchez-Alonso F, Belzunegui-Otano J. Key factors in optimizing the dose of Baricitinib in rheumatoid arthritis: a study based on routine clinical practice and its therapeutic implications [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/key-factors-in-optimizing-the-dose-of-baricitinib-in-rheumatoid-arthritis-a-study-based-on-routine-clinical-practice-and-its-therapeutic-implications/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/key-factors-in-optimizing-the-dose-of-baricitinib-in-rheumatoid-arthritis-a-study-based-on-routine-clinical-practice-and-its-therapeutic-implications/
