Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite systemic inflammatory cues, RA affects synovial joints variably. Tofacitinib is an oral JAK inhibitor for the treatment of RA. Previous post hoc analyses of data from ORAL Standard and ORAL Strategy showed differences in the degree/rate at which joints respond to tofacitinib ± MTX and adalimumab + MTX.1 However, clear patterns relating to specific agents were generally not seen, possibly as most patients (pts) received combination therapy with MTX. Here, we further characterize joint-specific responses to tofacitinib monotherapy and MTX using data from the Phase 3 study ORAL Start (NCT01039688).
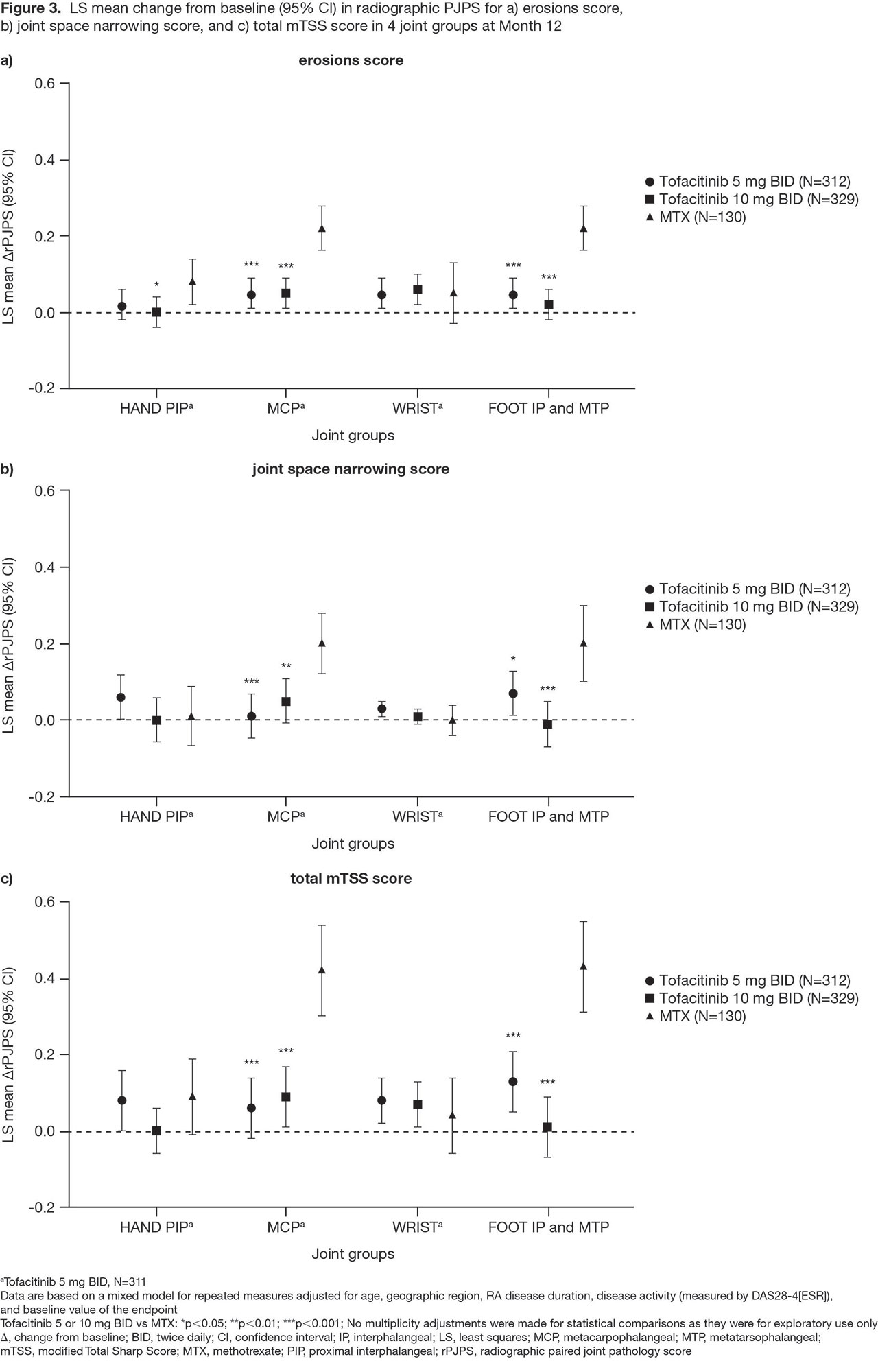
Methods: This was a post hoc analysis of Month (M)3, 6, and 12 data from ORAL Start in which pts with newly diagnosed, active RA received tofacitinib 5 or 10 mg twice daily (BID), or MTX (starting dose 10 mg/week and titrated up to 20 mg/week). A paired joint pathology score (PJPS), a combination of bilateral tender/swollen joint counts ranging from 0 (neither) to 4 (both sides tender/swollen), was calculated. Percentage changes from baseline in PJPS (%ΔPJPS) for each joint were presented in a heat map. To show treatment-specific effects at each joint, differences in %ΔPJPS for each tofacitinib dose, minus the MTX group, were calculated and presented in a further heat map. Joint damage in the hand PIP joints, MCP joints, wrist, and foot IP and MTP joints was assessed using the modified Total Sharp Score (mTSS). For each component of the mTSS composite score (erosion, joint space narrowing) and total score, a radiographic PJPS (rPJPS; combination of bilateral mTSS subscores in each joint group) was calculated and mean ΔrPJPS at M12 for each joint group presented. Statistical comparisons between tofacitinib vs MTX were performed using a repeated measures mixed model.
Results: Across treatment arms, all joints showed a treatment response (%ΔPJPS ranged from -95% to -39%), with responses generally improving over time (Figure 1). As previously reported,1 irrespective of treatment, the wrist, knee, and ankle responded less well than other joints. Differences in %ΔPJPS showed better responses for tofacitinib vs MTX in most joint locations, excepting some foot joints (Figure 2). Radiographic progression was minimal in all 4 joint groups (hand PIP joints, MCP joints, wrist, and foot IP and MTP joints) with both tofacitinib doses (Figure 3). The greater %ΔPJPS seen in the foot for MTX vs tofacitinib did not translate into improved radiographic outcomes at M12: ΔrPJPS in the foot were significantly worse for MTX vs tofacitinib across each endpoint (Figure 3), as were ΔrPJPS in the MCP joints.
Conclusion: Wide variation in RA joint-specific responses to tofacitinib 5 and 10 mg BID, and MTX, was seen in this post hoc analysis. Tofacitinib was generally more effective than MTX in all joints, except some foot joints. Enhanced efficacy in the distal lower limb has not been described with MTX before. Radiographic progression was minimal in the tofacitinib arms, while in the MTX arm, significantly more radiographic progression was observed in the MCP joints and foot.
- Ciurea A et al. Ann Rheum Dis 2019; In Press.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by K Woollcott of CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Frank-Bertoncelj M, Distler O, Killeen T, Kwok K, Wang L, Ospelt C, Ciurea A. Joint-specific Responses to Tofacitinib and Methotrexate in Rheumatoid Arthritis: A Post Hoc Analysis of Data from ORAL Start [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/joint-specific-responses-to-tofacitinib-and-methotrexate-in-rheumatoid-arthritis-a-post-hoc-analysis-of-data-from-oral-start/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-specific-responses-to-tofacitinib-and-methotrexate-in-rheumatoid-arthritis-a-post-hoc-analysis-of-data-from-oral-start/