Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Peripheral joint involvement in PsA varies. In RA, varied joint involvement may reflect site-specific differences in stromal cell transcriptome, including Janus kinase-signal transducer and activator of transcription (JAK-STAT). Tofacitinib, an oral JAK inhibitor for the treatment of PsA, has been associated with improvements in peripheral arthritis.1 Data on joint-specific responses in patients (pts) with PsA are sparse.2 This analysis describes patterns of peripheral joint involvement and response to tofacitinib or adalimumab (ADA) treatment in pts with active PsA.
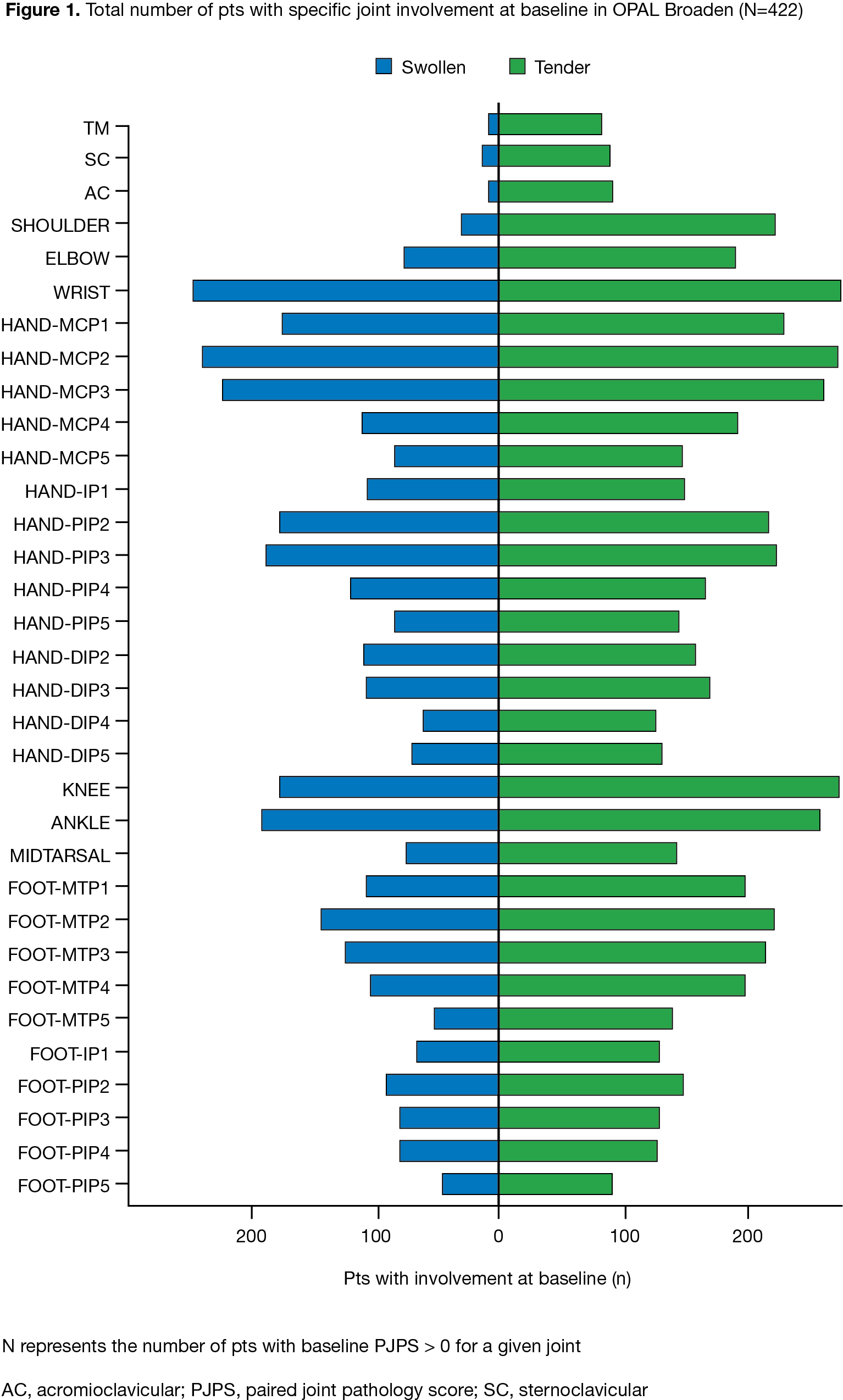
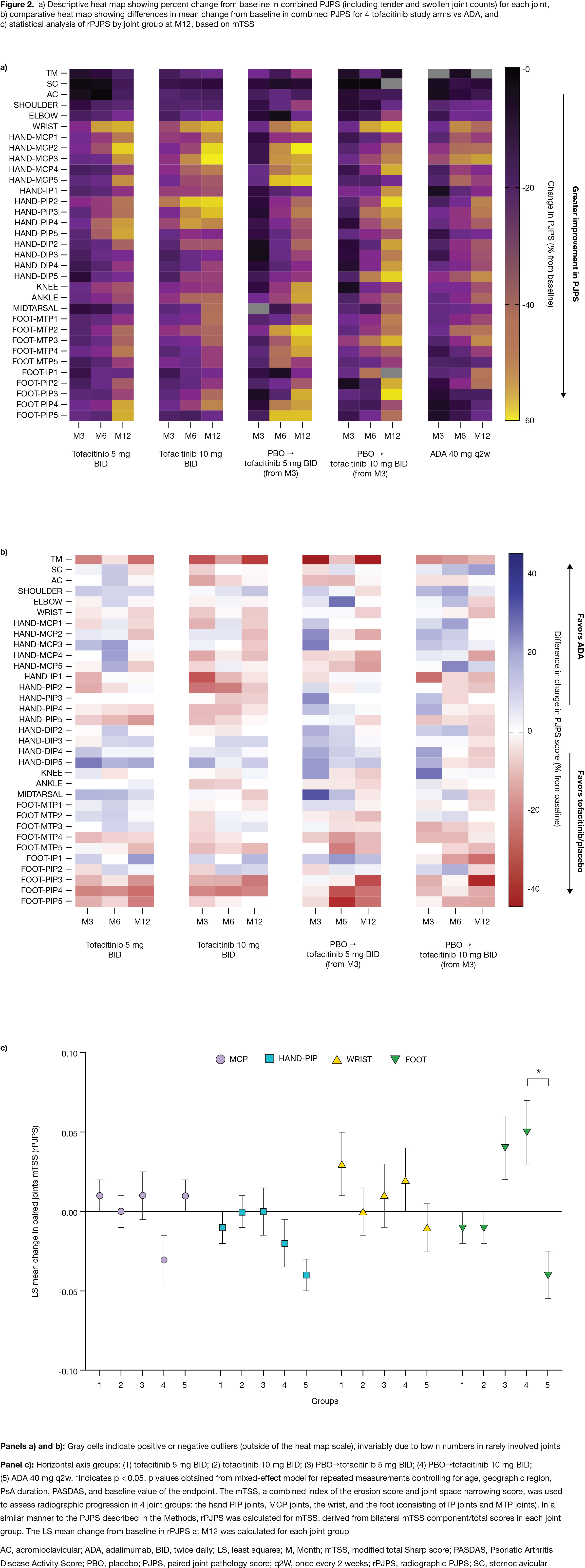
Methods: This post hoc analysis used data from OPAL Broaden (12 months; NCT01877668; N=422). Pts with PsA received tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg BID, ADA 40 mg subcutaneous injection every 2 weeks, or placebo (PBO) switching to tofacitinib 5 or 10 mg BID at Month (M)3; all pts received a single background conventional synthetic DMARD (75–88% of pts received MTX).1 OPAL Broaden was not designed to test non-inferiority/superiority between tofacitinib and ADA. Data at M3, 6, and 12 were included. A paired joint pathology score (PJPS), the sum of bilateral swollen/tender joint counts ranging from 0 (neither side swollen/tender) to 4 (both swollen and tender), was calculated. Percent change from baseline (%Δ) in PJPS for each joint and absolute differences in mean PJPS for each tofacitinib dose and PBO switch group at M3, 6, and 12 vs ADA were displayed as descriptive heat maps. Joint damage at M12 in the hand PIP, MCP, and wrist joints, and foot (IP and MTP joints), was assessed using components of the modified total Sharp score (mTSS). Statistical comparison of radiographic PJPS (rPJPS), derived from bilateral mTSS in each joint group, was performed using a mixed-effects model for repeated measurements.
Results: The baseline distribution of involved joints was typical for PsA (Fig 1).2 At M3, %ΔPJPS (improvement) was greater in joints below the knee with PBO. At M12, greater reductions in PJPS were observed with tofacitinib or ADA in the wrist, radial MCPs, and hand PIPs vs other joints (Fig 2a). At M12, greater reductions in PJPS were observed with ADA vs tofacitinib in the hand DIPs, and in the hand PIPs and feet (especially PIPs) with tofacitinib vs ADA (Fig 2b). Despite no major numeric differences in %∆rPJPS with tofacitinib vs ADA, greater radiographic progression in the feet was observed at M12 in pts switching from PBO to tofacitinib 10 mg BID at M3, vs ADA (p < 0.05) (Fig 2c). Various limitations preclude comparisons between tofacitinib and ADA.
Conclusion: We provide descriptive data on joint-level responses in pts with PsA receiving tofacitinib or ADA, and further evidence of a possible proximal/distal response gradient in MTX-treated arthritis.3 No consistent joint response patterns were observed with tofacitinib or ADA. A 3-month delay in advanced therapy initiation may result in radiographic progression in the feet.
1. Mease P et al. N Engl J Med 2017; 377: 1537-50.
2. Stekhoven D et al. Clin Rheumatol 2017; 36: 2035-43.
3. Frank-Bertoncelj M et al. Arthritis Rheumatol 2019; 71 (Suppl 10): Abs 1388.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by M Macfadyen, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Ciurea A, Killeen T, Micheroli R, Gassman N, Jo H, Kwok K, Kudlacz E, Distler O, Ospelt C, Frank-Bertoncelj M. Joint-specific Responses to Tofacitinib and Adalimumab in Patients with Psoriatic Arthritis: Post Hoc Analysis of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/joint-specific-responses-to-tofacitinib-and-adalimumab-in-patients-with-psoriatic-arthritis-post-hoc-analysis-of-a-phase-3-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-specific-responses-to-tofacitinib-and-adalimumab-in-patients-with-psoriatic-arthritis-post-hoc-analysis-of-a-phase-3-study/