Session Information
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Tanezumab, a monoclonal antibody that inhibits nerve growth factor, has been shown effective in the management of osteoarthritis (OA) pain.1,2 Due to the potential risk of rapidly progressive OA, recent phase 3 studies of subcutaneous tanezumab included a comprehensive prospective assessment of joint safety. The analyses summarized here include baseline population characteristics, adjudicated joint safety outcomes and sub-group analyses in the combined randomized, controlled, phase 3 OA tanezumab studies.
Methods: In study 1 (NCT02697773) patients received placebo, tanezumab 2.5 mg, or 2.5 mg then 5 mg (tanezumab 2.5/5 mg) over 16 weeks. In study 2 (NCT02709486) patients received placebo, tanezumab 2.5 mg, or tanezumab 5 mg over 24 weeks. In study 3 (NCT02528188) patients received tanezumab 2.5 mg, tanezumab 5 mg, or nonsteroidal anti-inflammatory drug (NSAID) over 56 weeks. Joint safety outcomes were adjudicated by a blinded external Adjudication Committee. The adjudicated composite joint safety endpoint (CJSE) included: primary osteonecrosis (ON), rapidly progressive OA type 1 (RPOA1) or type 2 (RPOA2), subchondral insufficiency fracture (SIF), or pathological fracture. Subgroups were cross-tabulated to investigate potential associations with outcomes descriptively with no formal statistical analysis.
Results: At baseline, maximum Kellgren-Lawrence (KL) grade of any joint and number of joints with KL grade ≥2 were similar across treatment groups (Table 1). A total of 451 patients were evaluated by the blinded adjudication committee; 145 were adjudicated to have a primary outcome which was a component of the CJSE: RPOA1 (69% 100/145), RPOA2 (17% 24/145), SIF (12% 18/145), and ON (2% 3/145) (Table 2). The proportion of patients with adjudicated CJSE was higher in tanezumab-treated patients relative to placebo or NSAID and the incidence increased with increasing dose of tanezumab. RPOA1 typically occurred in the knee and in joints with KL grade 2 or 3 OA at baseline, although there were four events in joints that were KL grade 0 at baseline (two each in the tanezumab 2.5 mg and 5 mg treatment groups). For RPOA2, the affected joint was approximately evenly split between the hip and knee with two patients having shoulder as the affected joint (one each in the tanezumab 5 mg and NSAID groups). Most RPOA2 events occurred in joints with KL grade 3 or 4 OA at baseline. RPOA2 was more often associated with a total joint replacement than RPOA1. Multiple subgroups were investigated to assess potential associations with CJSE, with possible associations noted for baseline disease severity and adverse events of arthralgia and joint swelling (Table 3).
Conclusion: Patients enrolled in the combined phase 3 OA tanezumab studies had moderate to severe OA. The proportion of patients with adjudicated CJSE was higher in tanezumab-treated patients relative to placebo or NSAID and the incidence increased with increasing dose of tanezumab. Subgroup analyses did not reveal an association of CJSE with efficacy response.
 Table 1. Baseline disease characteristics, n (%)
Table 1. Baseline disease characteristics, n (%)
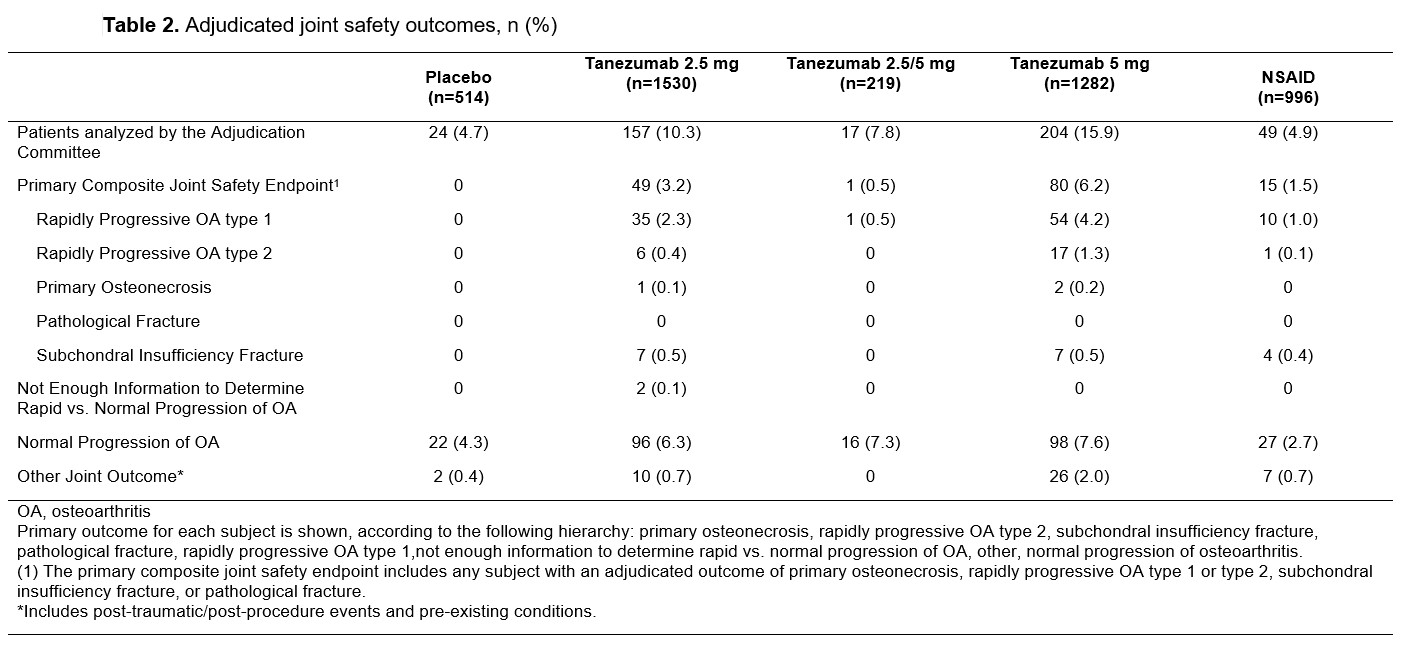 Table 2. Adjudicated joint safety outcomes, n (%)
Table 2. Adjudicated joint safety outcomes, n (%)
 Table 3. Subgroups analyzed for potential association with adjudicated composite joint safety endpoint
Table 3. Subgroups analyzed for potential association with adjudicated composite joint safety endpoint
To cite this abstract in AMA style:
Carrino J, McAlindon T, Vignon E, Brown M, Burr A, Fountaine R, Pixton G, Viktrup L, West C, Verburg K. Joint Safety with Tanezumab: Integrated Analyses from Randomized Controlled Phase 3 Studies in Patients with Osteoarthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/joint-safety-with-tanezumab-integrated-analyses-from-randomized-controlled-phase-3-studies-in-patients-with-osteoarthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-safety-with-tanezumab-integrated-analyses-from-randomized-controlled-phase-3-studies-in-patients-with-osteoarthritis/
