Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is an anti-malarial medicine and an immunomodulator to treat autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Clinical beneficial evidence of HCQ includes anti-thrombotic effects, anti-arthritic effects seen in short term, and disease-stabilizing effects to inhibit the relapse of SLE, renoprotective effect, better life prognosis as long-term effects. On the other hand, there are also non-negligible adverse effects of HCQ such as retinal and renal toxicity. It is known that HCQ cellular cytotoxicity is exerted by inhibiting autophagy and lysosomal enzymes. However, it still remained to be clarified how interference of lysosomal-autophagy pathway by HCQ leads to cellular cytotoxicity. Janus kinase (JAK) was previously reported to be involved in lysosome biogenesis in visceral glomerular epithelial cells (podocytes), hence we pursuit the possibility that JAK inhibitors (JAKis) could reduce cellular cytotoxicity of HCQ in vitro in this study.
Methods: Luminescent-based cell viability plate assay (CellTiter-GloⓇ Luminescent Cell Viability Assay, Promega, USA) was employed to quantify HCQ-induced cell death. Human retinal pigment epithelial cell line (ARPE-19), mouse podocyte cell line (AI), and human podocyte cell line (BLAK) were used as HCQ-induced retinopathy and nephropathy in vitro models to test HCQ cellular toxicity. ARPE-19 was plated on non-coated 96 well plates and AI and BLAK were plated on collagen 1-coated 96 well plates (day 1). ARPE-19 was pretreated with a series concentrations of upadacitinib, and AI and BLAK were pretreated with a series concentrations of baricitinib for 48 hours (day 3, 4). On Day 5, 10 to 80 μg/mL of HCQ was added to these cell lines to induce cell death, and the culture was continued for 4 to 9 days, until cells were subject to viability assay. One-way ANOVA was done using Prism 9 for macOS for statistical analysis.
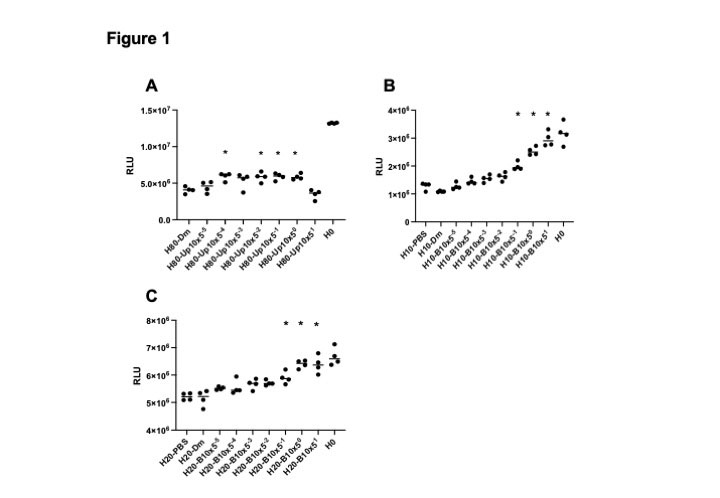
Results: A series of HCQ concentrations ranging from 0.009 to 80 μg/mL were tested to decide the most appropriate concentration to kill cells, resulting in 80 μg/mL for ARPE-19, 10 μg/mL for AI and 20 μg/mL for BLAK. Upadacitinib at the concentration of 16 nM~10 μM siginificantly inhibited 80 μg/mL HCQ-induced ARPE-19 cell death (Figure 1A). In AI and BLAK (Figure 1B and 1C), 2 to 50 μM of baricitinib significantly inhibited 10 μg/mL and 20 μg/mL HCQ-induced cell death, and protective trend was also seen in the lower concentrations such as 16, 80 and 400 nM of baricitinib. Maximam plasma concentration (cMAX) of 8 mg baricirinib in adults is 107 ng/mL (280 nM), and that of 15 mg upadacitinib in adults is 61 ng/mL (156 nM). JAKi protective effects on HCQ cellular toxicity in these experiments were observed at the clinically-relevant concentration less than cMAX of each JAKi tested.
Conclusion: JAKis counteract celluar cytotoxicity of HCQ in a human retinal pigment epithelial cell line, a mouse podocyte cell line and a human podocyte cell line in vitro. JAK inhibitors might reduce the incidence of retinopathy and that of nephropathy in patients treated with HCQ.
Abbreviations in the Y axis: RLU relative light unit. Asterisks statistically significant in one-way ANOVA, P<0.01.
To cite this abstract in AMA style:
Kajiyama H, Aizaki Y, Yazawa H, Yokota K, Araki Y, Akiyama Y, Mimura T. JAK Inhibitors Counteract Cellular Toxicity of Hydroxychloroquine in Vitro [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/jak-inhibitors-counteract-cellular-toxicity-of-hydroxychloroquine-in-vitro/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/jak-inhibitors-counteract-cellular-toxicity-of-hydroxychloroquine-in-vitro/