Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Down syndrome associated arthritis (DA) is a challenging form of inflammatory arthritis that typically is more erosive and has a greater requirement for biologic agents than JIA (Juvenile Idiopathic Arthritis). There remains a paucity of information on potential therapeutic agents for this aggressive condition. Studies have hypothesized the presence of immune dysregulation due to interferon (IFN) signalling hyperactivation in patients with Down Syndrome (DS). As Janus kinase (JAK) plays an important role in IFN signalling; using JAK inhibitors such as Tofacitinib which blocks JAK1/JAK 3 pathway therefore block IFN hyperactivity with therapeutic benefit in DA . We present our experience to date of DA patients treated with Tofacitinib.
Methods: All patients with DA treated with Tofacitinib between August 2021 to August 2022 were included. Data was collected at baseline with plan to be collected at 3- and 6-months post treatment but due to COVID-19 backlog in clinic it was collected at 1-3 months for initial visit and then 6-8 months for 2nd clinic visit. The total active joint count, use of non-steroidal anti-inflammatory medication (NSAID), exercise endurance, infection episodes, side effects of medication, overall parental satisfaction with medication and inflammatory markers at each assessment were documented.
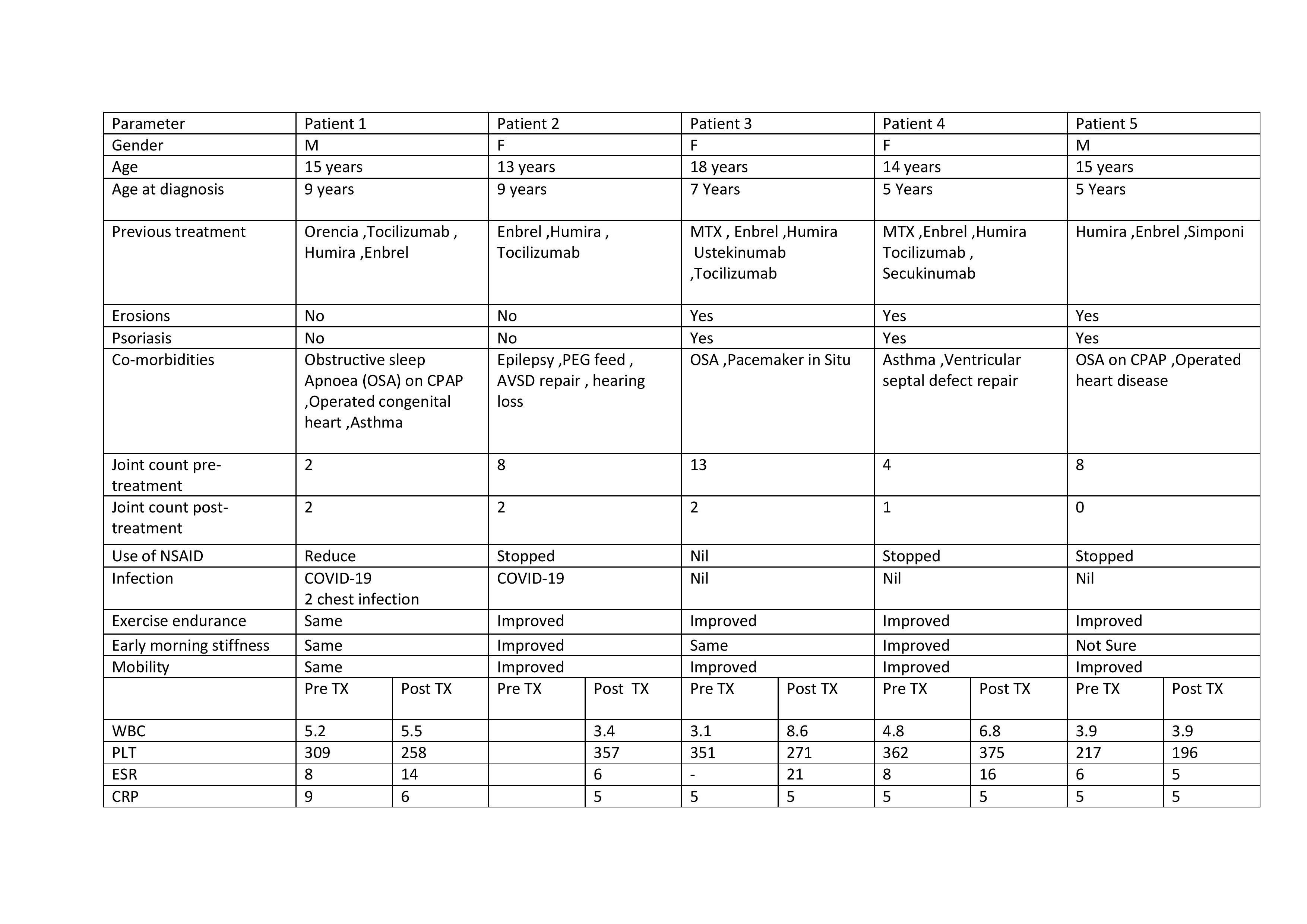
Results: A total of 5 patients (2 Male, 3 females), aged between 13-18 years were identified. All patients were of European ethnicity. The duration since diagnosis with DA ranged between 4-11 years. All 5 patients used minimum of 3 biological agents with no or minimal disease control and 2 patients failed trial of Methotrexate in addition to biological therapy. 3 patients (60%) had diagnosis of erosive arthritis and psoriasis. 4 out of 5 patients had improvement in the joint counts (80%). All patients had reduced amount of NSAID use with 3 patients having stopped pain medication altogether. Improvement in exercise endurance noticed by family in 4 patients. There was no reported increase in infection episodes. 2 patients had slightly higher ESR at 2nd follow up visit compared to baseline blood while WBC count, haemoglobin, Platelets, CRP remained unchanged. 2 out of 3 patients with psoriasis had resolution of the rash with Tofacitinib. An overall improvement in clinical status noted by parents was reported between 4-12 weeks after starting the treatment and all 5 families (100%) agreed that Tofacitinib was the most beneficial biologic agent to date.
Conclusion: Down Syndrome associated arthritis is a relatively newly described condition that affects approximately 1in 50 with DS. It is an aggressive form of arthritis that does not respond as well as JIA to conventional medications. To our knowledge this is the first case series outlining the use of JAK inhibitors. Although numbers included were small, results were favourable with promising improvements reported and no significant major side effects reported. A larger and prospective study is required to prove the beneficial role of JAK inhibitor in managing patients with DA and changes treatment approach in Down Associated arthritis.
To cite this abstract in AMA style:
Alkandari A, killeen O. JAK Inhibition in down Syndrome Associated Arthritis (DA) – Our Experience to Date with Tofacitinib in 5 Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/jak-inhibition-in-down-syndrome-associated-arthritis-da-our-experience-to-date-with-tofacitinib-in-5-patients/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/jak-inhibition-in-down-syndrome-associated-arthritis-da-our-experience-to-date-with-tofacitinib-in-5-patients/