Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: IL-17 inhibition demonstrates efficacy in multiple disease domains in psoriatic arthritis. Izokibep is a unique IL-17A inhibitor with high IL-17A binding affinity (KD= 0.3 pM), small molecular size (18.6 kDa), and an albumin attachment site. Week 16 data showed ACR50 of 52% and enthesitis resolution rates of 88%. We report data from baseline to 46 weeks in this phase 2 PsA trial1 on arthritis and skin composite efficacy endpoints and longer-term safety.
Methods: Izokibep doses of 80 mg Q2W or 40 mg Q2W were evaluated to 46 weeks or study termination. The original placebo arm switched to 80 mg Q2W at week 16 in this multicenter trial (NCT04713072). Once week 16 data were available, this trial was terminated to further examine the effective dose range of izokibep in a next P2b/3 trial. The results include as observed analysis. Eligible patients met CASPAR criteria, with ≥3 swollen and ≥3 tender joints, and prior failure/insufficient response to NSAIDs, csDMARDs or TNF inhibitors.
Results: 135 patients were randomized 1:1:1: 44 to 40 mg Q2W, 47 to 80 mg Q2W, 44 to placebo later switched to 80 mg Q2W at week 16. At week 32, 96 of 102 eligible patients had measured results and at week 46, 59 of 62 patients did. Baseline mean age was 49 (SD 12), BMI 29 (5), PsA duration 7 (8) years, SJC 10 (7) and TJC 17 (10), DAPSA 47 (22), and PsAID-9 5.9 (1.8). Mean PASI was 10 (6) in those patients with BSA >3%, and in those with enthesitis, mean LEI was 1.5 (0.5) and SPARCC was 3.4 (2.8). 13% used prior TNF inhibitors.
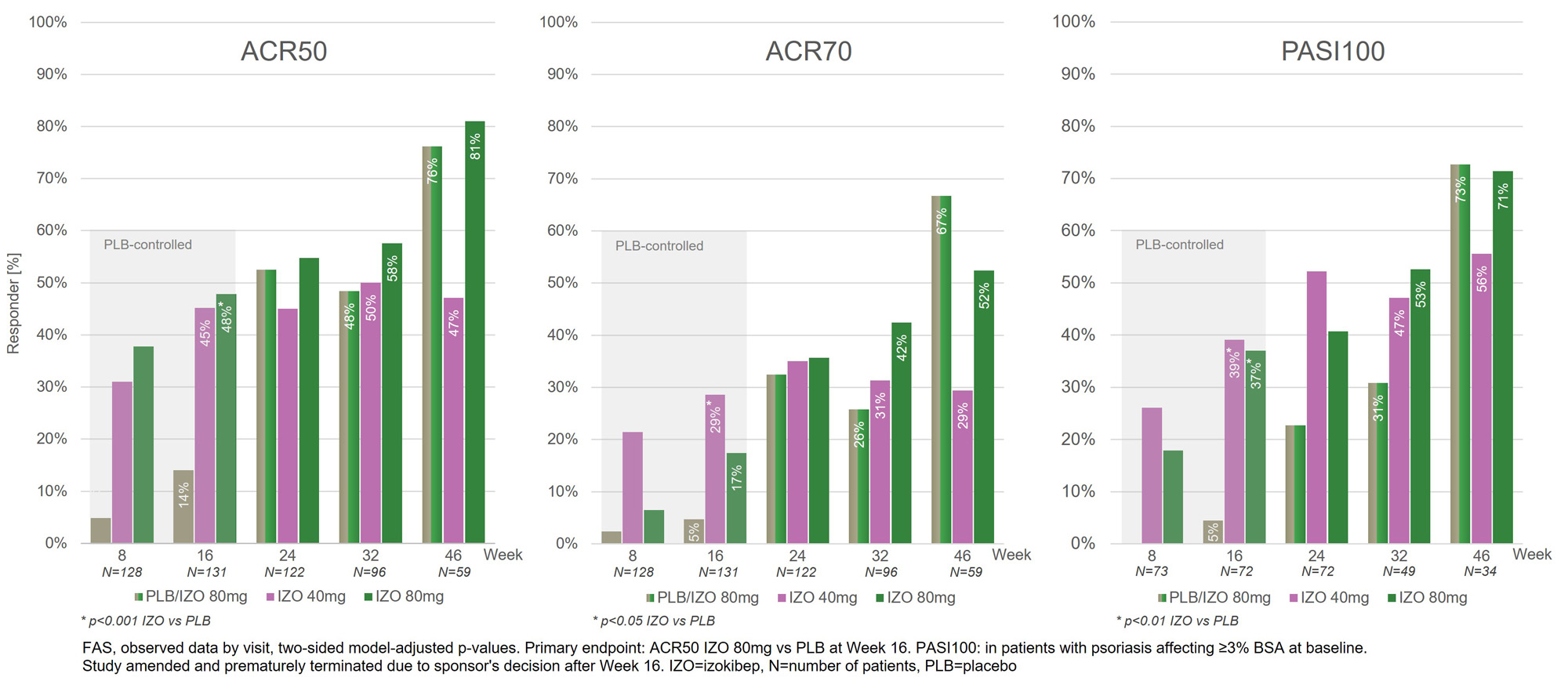
Beyond week 16, the 80 mg groups continued to improve, while the 40 mg group remained largely stable (Figure 1).
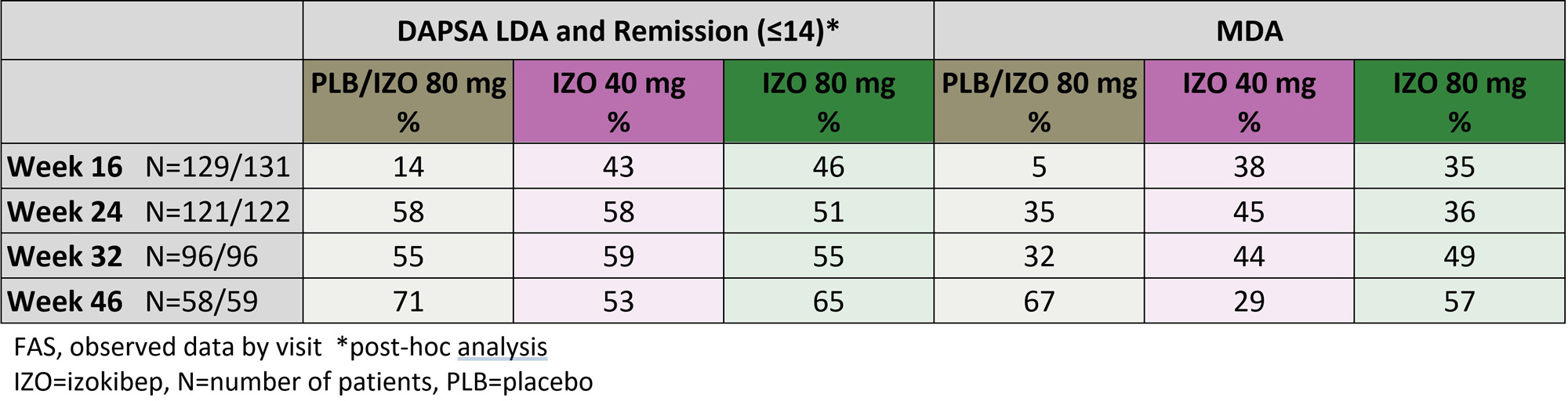
Most patients in the 80 mg group and the placebo/80 mg switchers achieved DAPSA low disease activity/remission and minimal disease activity (MDA) thresholds through week 46 (Table).
High LEI enthesitis resolution rates on 80 mg until week 16 were maintained while SPARCC enthesitis resolution progressed through week 46 (Figure 2). Mean PsAID-9 scores improved to week 46, to a mean 2.3 (2.0) on 80 mg, 2.2 (2.1) in the placebo/80 mg switchers and remained largely stable on 40 mg after 16 weeks, with mean 3.2 (2.5).
Safety over the interval from week 16 to 46 remained unchanged. The most frequent adverse events were injection site reactions (14.5%) and injection site erythema (12.2%), with 1 patient discontinuing for ISR. Nasopharyngitis occurred in 6.9%, and headache and backpain occurred in 5.3% each. AEs were mostly mild and balanced across treatment groups. No Candida or fungal infections were observed from weeks 16 to 46.
Conclusion: Izokibep 80 mg demonstrated high levels of disease control with ACR70 in 52%, PASI100 in 71% and enthesitis complete resolution in 89% at week 46. Izokibep remained well tolerated, with no dose related adverse events and a safety profile generally consistent with approved IL-17A inhibitors.
References
1Behrens, F et al. ACR 2022. Abstract 1597. Arthritis Rheumatol. 2022; 74 (suppl 9).
To cite this abstract in AMA style:
Mease P, Taylor P, de Vlam K, Peloso P, Lertratanakul A, Wetzel D, Brun N, Wiens B, Brandt-Juergens J, Drescher E, Dokoupilova E, Rowińska-Osuch A, Abdel-Kader Martin N, Behrens F. Izokibep Demonstrates Major Disease Control on ACR70, PASI100 and Enthesitis Resolution in Patients with Active Psoriatic Arthritis Treated Through 46 Weeks [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/izokibep-demonstrates-major-disease-control-on-acr70-pasi100-and-enthesitis-resolution-in-patients-with-active-psoriatic-arthritis-treated-through-46-weeks/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/izokibep-demonstrates-major-disease-control-on-acr70-pasi100-and-enthesitis-resolution-in-patients-with-active-psoriatic-arthritis-treated-through-46-weeks/