Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Izokibep is a novel small protein IL-17A inhibitor, unique for its high IL-17A binding affinity, a small size at 18.6 kD and albumin binding site enabling access in inflamed tissues and two-week half-life. IL-17A is key in psoriatic arthritis (PsA) pathogenesis and its inhibition is known to improve quality of life. Here, we report results of izokibep treatment to week 46 on patient-reported outcomes (PROs), following a 16-week placebo-controlled period1. PRO endpoints include PsAID-9, HAQ-DI, TSQM-9, and patient reported pain, itch, and global disease activity.
Methods: Izokibep doses of 80 mg Q2W or 40 mg Q2W were evaluated to 46 weeks or study termination. The original placebo arm continued to 16 weeks, then switched to 80 mg Q2W in a multicenter trial (NCT04713072). This study was terminated once 16-week results were available to continue to explore the effective dose range in a next P2b/3 trial. The results include as observed analysis evaluating change from baseline to weeks 16, 32 and 46. Patients met CASPAR criteria and had ≥3 swollen (SJC) and ≥3 tender joints (TJC) on a 66/68 joint count and inadequate response to previous NSAIDs, csDMARDs, or TNF inhibitor therapy.
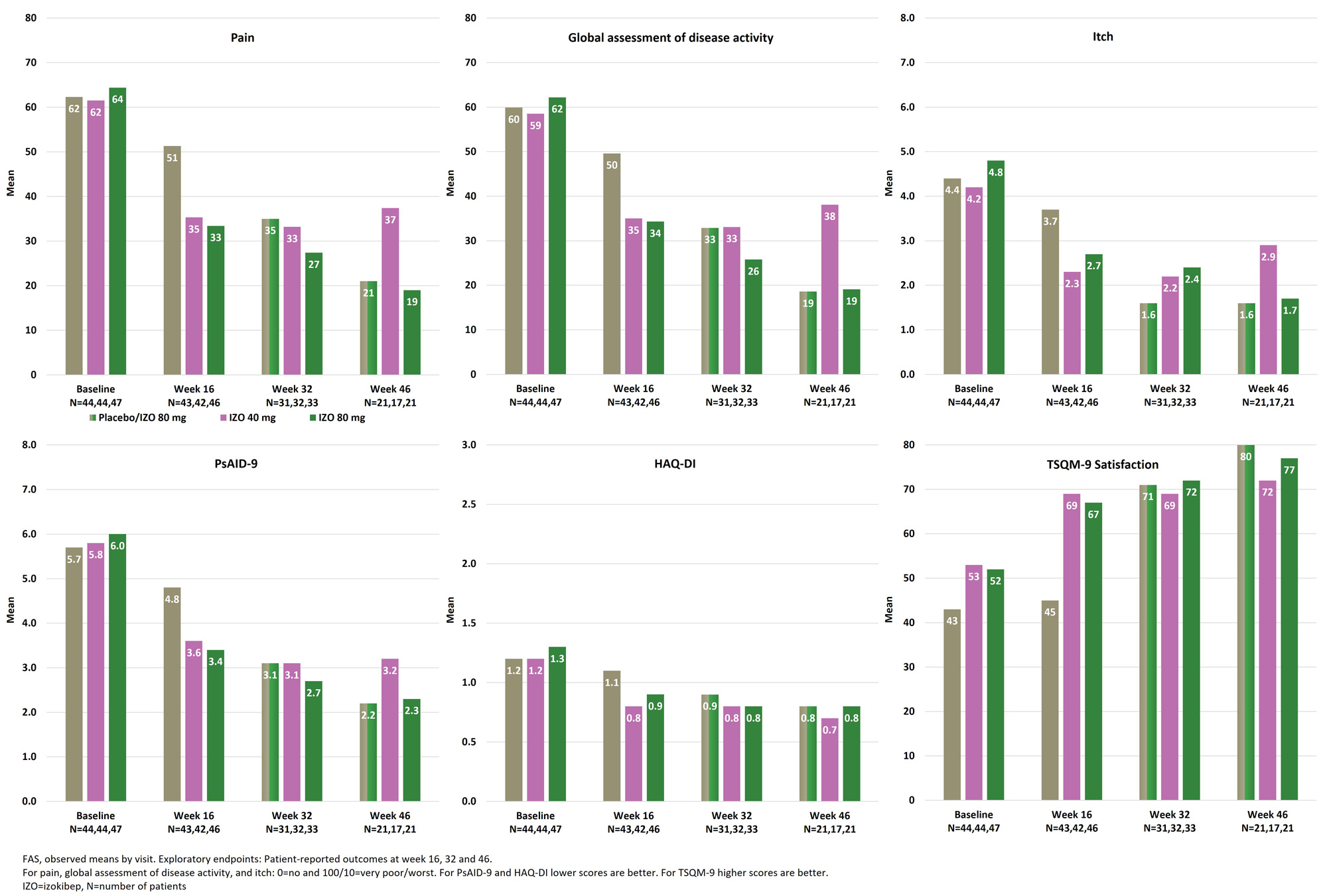
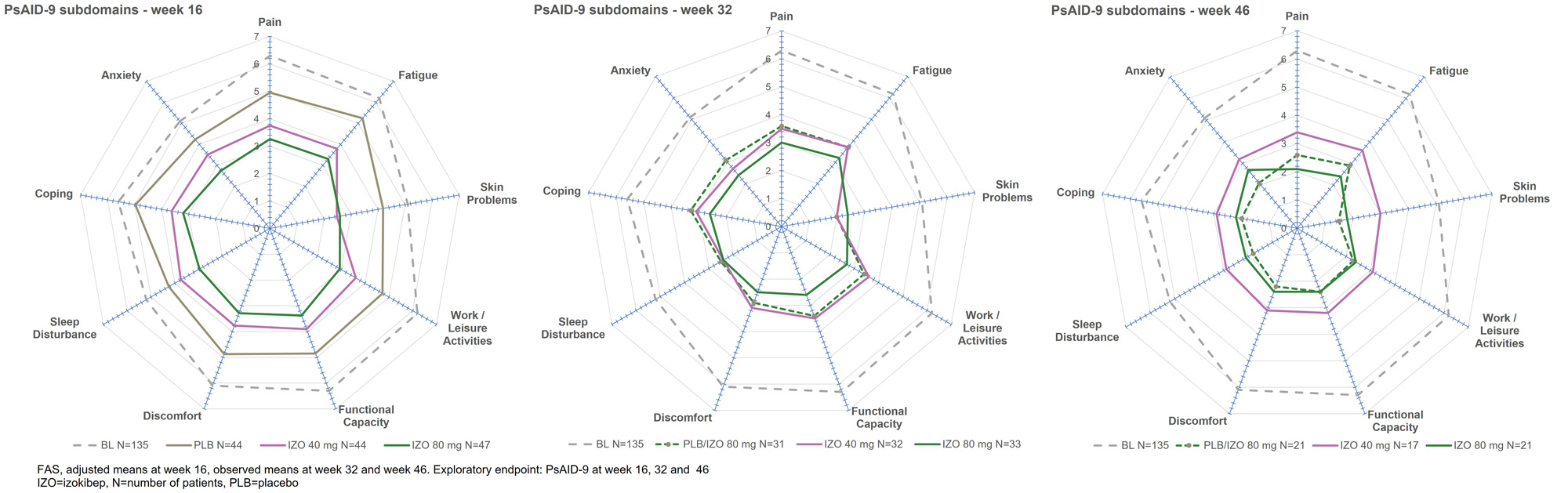
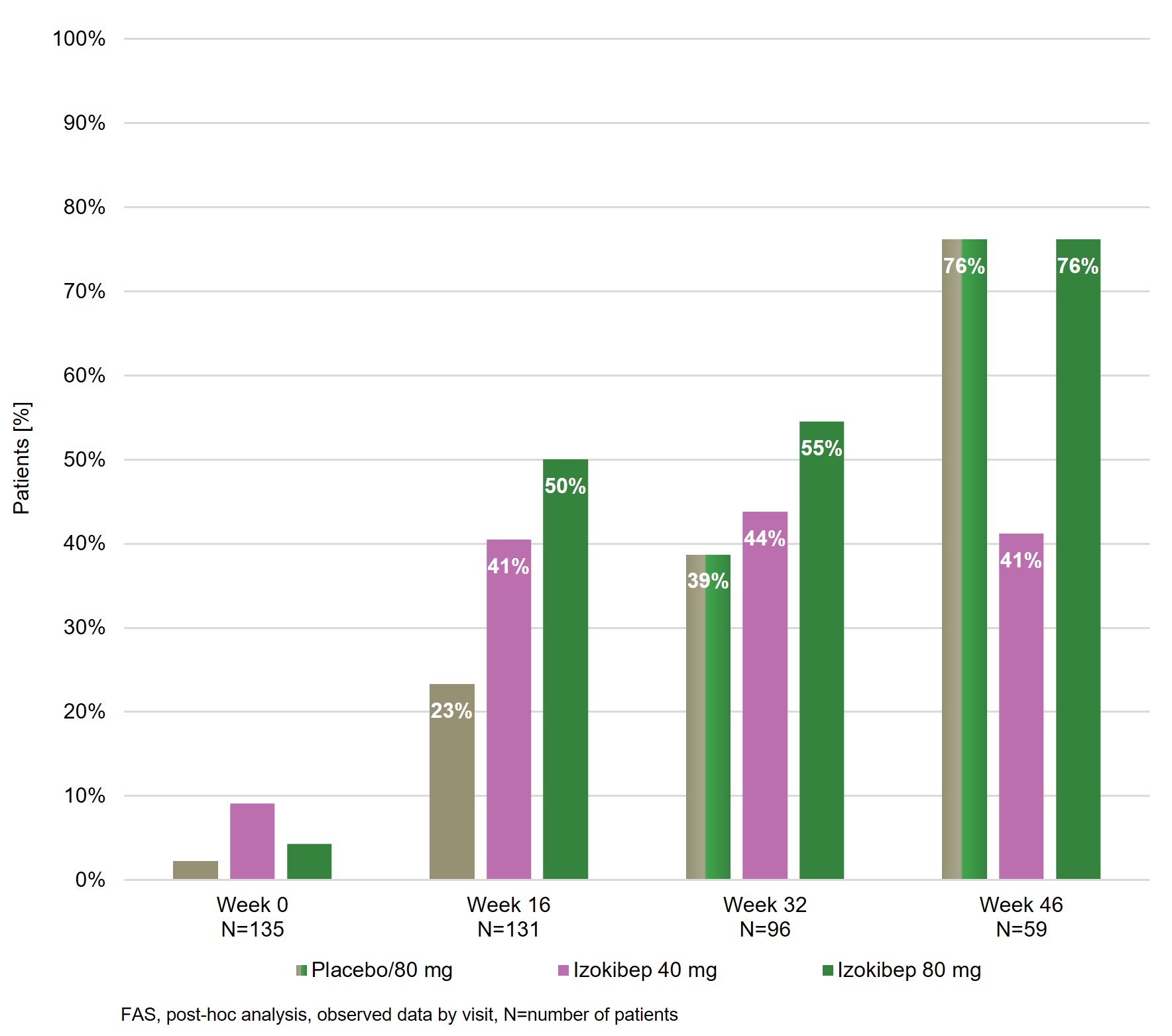
Results: 135 patients were randomized 1:1:1 to izokibep 40 mg, 80 mg or placebo to week 16 when placebo transitioned to 80 mg Q2W. Patients had a mean age of 49 (SD 12) years, a mean BMI of 29 (5) kg/m2, a mean PsA disease duration of 7 (8) years, a mean SJC of 10 (7) and TJC of 17 (10). At week 32, 96 of 102 eligible patients had evaluable data and at week 46, 59 of 62 eligible patients had evaluable data with similar distribution across groups. Clinically important improvements were evident for pain, patient global disease activity, itch, PsAID-9, treatment satisfaction on the TSQM-9 for those starting on 80 mg at baseline as well as those switching to 80 mg at week 16. The 80 mg dose showed improvements from week 16 to 46, whereas the 40 mg dose was largely stable (Figure 1). Consistent improvements across all PsAID-9 subdomains were evident for the 80 mg doses at week 32 and week 46 (Figure 2). Pain responders, defined as achieving patient reported pain < 30/100, improved from weeks 16 to 32 and 46 for those starting with 80 mg and those switching to 80 mg at week 16 while the 40 mg dose remained largely stable (Figure 3). From week 16 to week 46, the most frequently reported adverse events were injection site reactions, injection site erythema and nasopharyngitis which were distributed similarly across groups. One patient dropped out for ISR over this interval. There were no dose-related infection risks.
Conclusion: Izokibep led to continuing improvements across all PROs from baseline to weeks 16, 32 and 46 with greater improvements in the 80 mg dose groups. The GRAPPA validated PsAID-9 showed clinically important improvements in patient reported quality of life across all subdomains over weeks 16, 32 and 46. More than 70% of patients achieved marked pain control. Izokibep was generally well tolerated with no new safety issues to week 46.
References
1Taylor, P C et al. ACR 2022. Abstract 0199. Arthritis Rheumatol. 2022; 74 (suppl 9).
To cite this abstract in AMA style:
Taylor P, de Vlam K, Mease P, Peloso P, Wetzel D, Lertratanakul A, Brun N, Wiens B, Brandt-Juergens J, Drescher E, Dokoupilova E, Rowińska-Osuch A, Abdel-Kader Martin N, Behrens F. Izokibep, a Unique IL-17A Inhibitor, Improves Patient-Reported Outcomes in Patients with Active Psoriatic Arthritis up to Week 46 – Phase 2 RCT Results [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/izokibep-a-unique-il-17a-inhibitor-improves-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-up-to-week-46-phase-2-rct-results/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/izokibep-a-unique-il-17a-inhibitor-improves-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-up-to-week-46-phase-2-rct-results/