Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Izokibep is a unique IL-17A inhibitor with high potency and small molecular size designed to overcome the limitations of monoclonal antibodies. IL-17 is a key driver of the PsA disease process, which greatly impacts patients’ physical function, vitality, social participation, mood, and quality of life. Here, we report 16-week results of izokibep on patient-reported outcomes (PROs) in adult patients with active PsA.
Methods: A prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-groups, dose-finding trial was performed evaluating izokibep 80 mg or 40 mg Q2W administered subcutaneously versus Placebo until Week 16. Patients met CASPAR criteria and had ≥3 swollen and ≥3 tender joints of the 66/68 joint count, and an inadequate response to previous NSAIDs, csDMARDs, or TNF inhibitor therapy. Secondary endpoints studying PROs included the SF-36, HAQ-DI, PsAID-9, DLQI, TSQM-9, and single-item scales for pain, patient-reported global disease activity, and itch. A priori statistical analyses were carried out on all studied PROs with posthoc analyses addressing minimal clinically important differences (MCIDs) for HAQ-DI, PsAID-9, and DLQI. ClinicalTrials.gov registration at NCT04713072.
Results: There were 135 patients randomized in 28 sites between June 2020 and July 2021. At baseline, patients had a mean age of 48.5 (SD 12.0) years, a mean PsA disease duration of 7.1 (SD 7.8) years, PSO duration of 18 (SD 14) years, SJC of 9.9 (SD 6.6), TJC of 16.7 (SD 10.4), HAQ-DI of 1.3 (SD 0.6), PsAID of 5.9 (SD 1.8), VAS pain of 62.8 (SD 19.8), VAS global disease activity of 60.2 (SD 20.3), and NRS itch of 4.5 (SD 2.6). Eighty percent were treated with one concomitant csDMARD. During the COVID-19 pandemic adherence to study visits (98%) and treatment compliance were almost complete (mean 7.9 of 8 IMP injections).
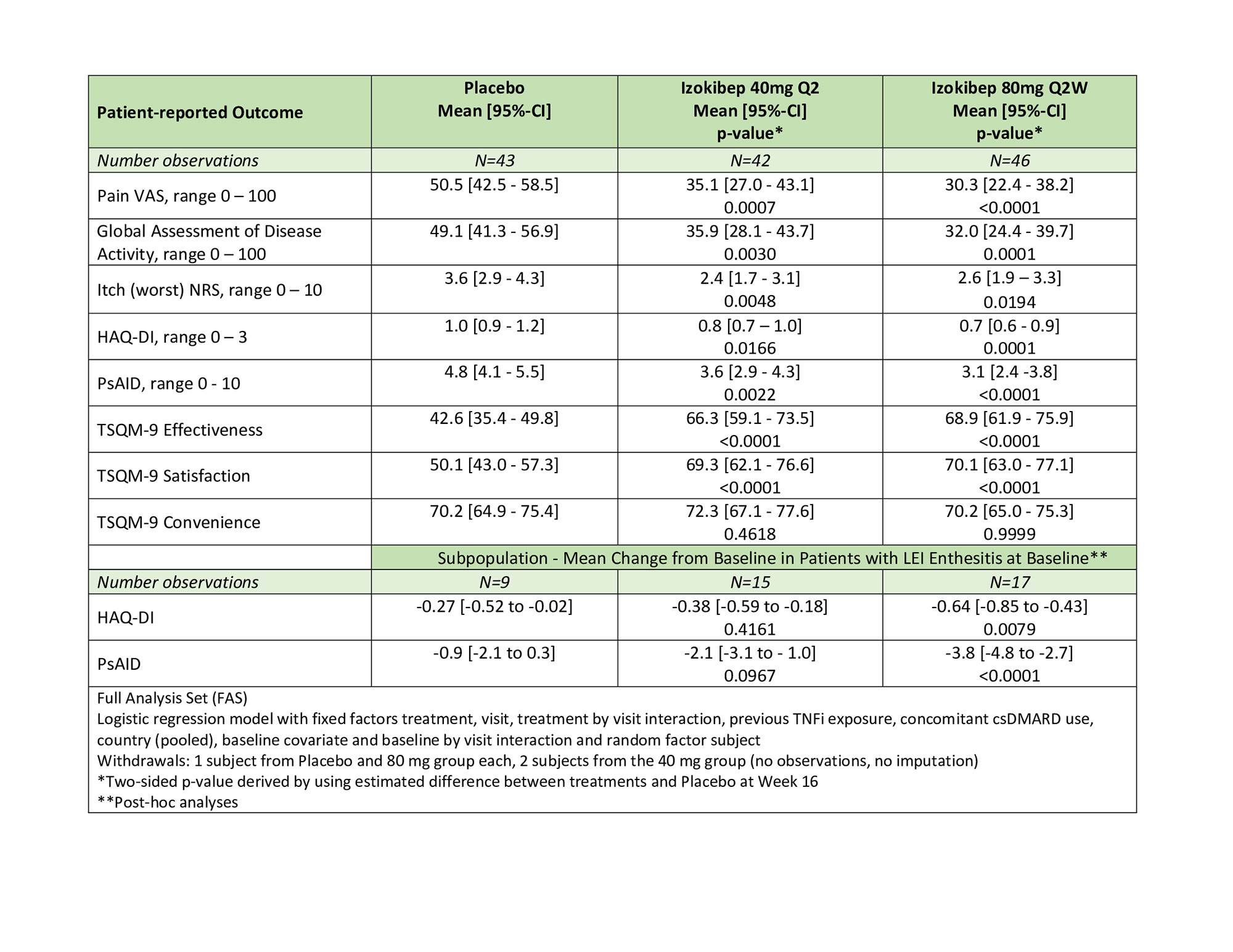
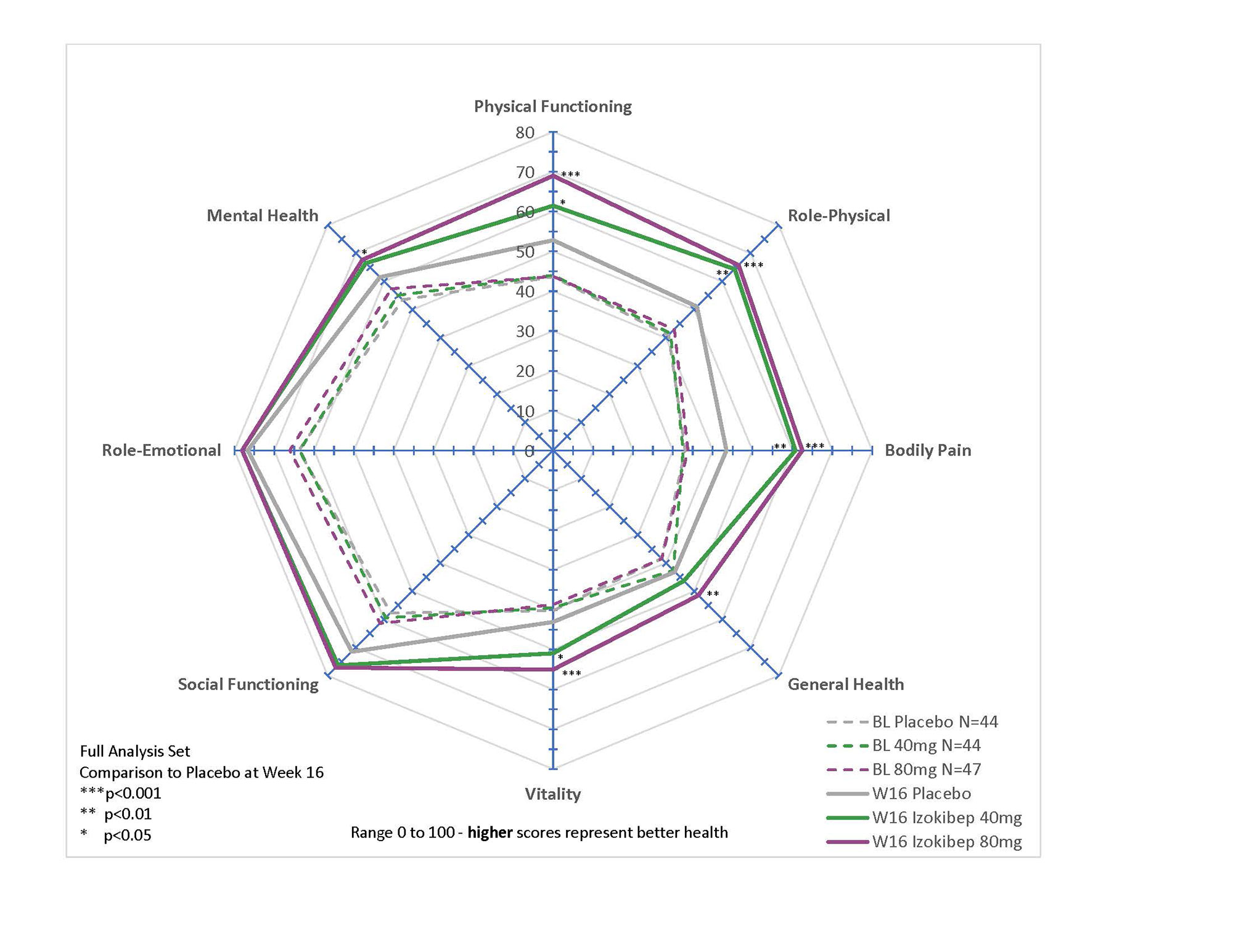
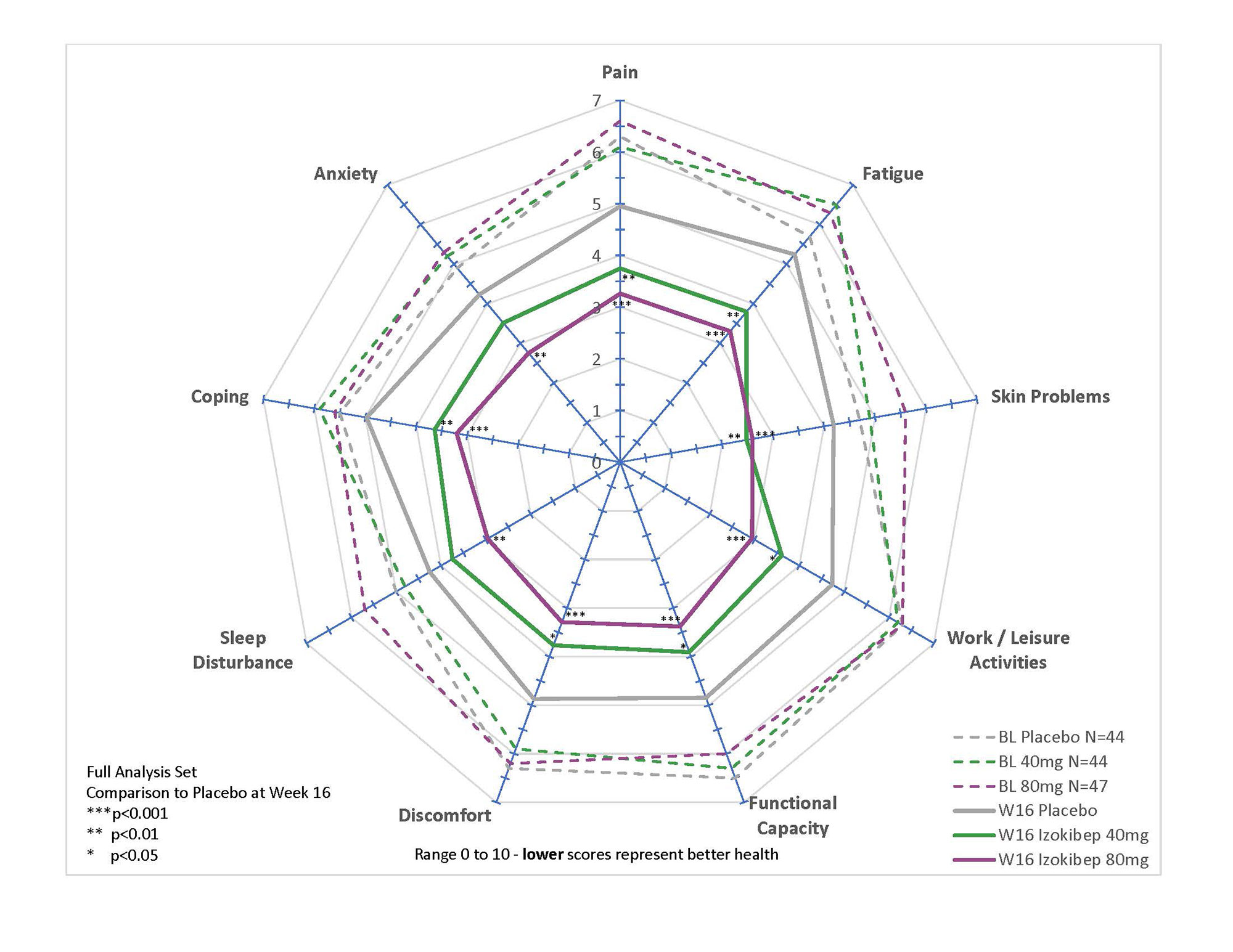
All studied PROs revealed significant and clinically meaningful improvements with large treatment effects demonstrated for PsAID, pain, global disease activity, and TSQM (Table 1). All SF-36 subdomains, apart from Role-Emotional and Social Functioning, improved in a dose-dependent manner with prominent effects on Bodily Pain, Role-Physical, Physical Functioning, and Vitality (p < 0.001 for 80 mg; Figure 1). All 9 PsAID subdomains improved significantly for the 80 mg group (p< 0.01; Figure 2), with notable improvements in sleep, pain, and function. A DLQI ≤ 5 was reached by 80% treated with 80 mg, 81% with 40 mg, and 63% treated with Placebo.
On HAQ-DI, the percent reaching MCID ( >0.35) was 54% (80 mg), 43% (40 mg) versus 26% (Placebo) of patients; PsAID MCID (≥3) was achieved by 41% (80 mg), 31% (40 mg) versus 12% (Placebo). In the sub-population (43/135) with Leeds Enthesitis Index (LEI) at baseline, the differential effects on PsAID were more marked with 53% (80mg), 27% (40 mg), and 0% (Placebo).
Conclusion: Izokibep demonstrated a clinically meaningful, dose-dependent improvement across studied PROs, including SF36 and PsAID subdomains, in patients with active PsA, with notable improvements in sleep, pain, and function. Patients with baseline enthesitis had an even greater benefit.
To cite this abstract in AMA style:
Taylor P, Behrens F, Mease P, Wetzel D, Peloso P, Brun N, Wiens B, Brandt-Juergens J, Drescher E, Dokoupilova E, Rowińska-Osuch A, Abdel- Kader Martin N, de Vlam K. Izokibep, a Novel IL-17A Inhibitor, Improves Patient-reported Outcomes – 16-Week Results from a Placebo-controlled Phase 2 Study in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/izokibep-a-novel-il-17a-inhibitor-improves-patient-reported-outcomes-16-week-results-from-a-placebo-controlled-phase-2-study-in-patients-with-active-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/izokibep-a-novel-il-17a-inhibitor-improves-patient-reported-outcomes-16-week-results-from-a-placebo-controlled-phase-2-study-in-patients-with-active-psoriatic-arthritis/